Coronavirus Disease 2019 - COVID 19
COVID-19: Management
Treatment
Laboratory investigation for proven COVID 19 patients
|
At Admission |
CBC, RFT, LFT, CRP, RBS, S.electrolytes, ECG, Pulse oximetry. |
|
If clinically Indicated |
Portable CXR, HIV, HBsAg, HCV, D-Dimer, Ferritin, LDH, CPK, procalcitonin, Blood culture, TROP T/I,HRCT Thorax |
|
To repeat Every 48 hours if clinically deteriorating. |
CBC, Creatinine, AST/ALT, CRP, LDH, CPK, Ferritin, D Dimer. |
|
For Immunocompromised patients eg Transplant recipients, HIV |
Tests to rule out opportunistic infections like Mycobacterium tuberculosis, pneumocystis jiroveci etc |
Identification of high risk patients
|
Co morbidities |
Clinical assessment |
Laboratory values |
|
Uncontrolled diabetes [HbA1C >7.6% |
Hypoxia - SpO2 < 94 % on room air |
CRP > 100 mg /L |
|
Hypertension |
Tachycardia PR > 125/min |
CPK > twice upper limit of normal |
|
Cardiovascular disease |
Respiratory distress RR > 24/min |
Ferritin > 300mcg/L |
|
Preexisting pulmonary disease |
Hypotension BP < 90systolic, 60mm Hg Diastolic |
LDH > 245 U /L |
|
CKD |
Altered sensorium |
TROP T elevation |
|
CLD |
PAO2/FiO2< 300 mm of Hg |
D Dimer > 1000ng/ml |
|
On immunosuppressives/biologicals | ||
|
HIV CD4 <200 / congenital immunodeficiency disorders |
Multi organ dysfunction | |
|
Age > 65 yrs |
*ALC < 0.8 | |
|
BMI >30 |
#NLR >3.13 |
*ALC - Absolute lymphocyte count #NLR - Neutrophil lymphocyte ratio [NLR -should be calculated prior to steroid administration]
CT Guided approach to diagnosis in suspected COVID 19 patient
In symptomatic patients with suspected COVID - 19, HRCT thorax may be considered for diagnosis of COVID -19 when initial RT-PCR testing is negative.CT guided approach should be used in RTPCR negatives cases with high clinical index of suspicion of COVID - 19.
|
Imaging classification |
Rationale |
CT appearance |
|
Typical appearance |
Commonly reported imaging features of greater specificity for COVID 19 pneumonia |
Peripheral bilateral GGO with or without consolidation or visible intra lobular lines (‘crazy paving') Multifocal GGO of rounded morphology with or without consolidation or visible intra lobular lines (‘crazy paving') Reverse halo sign or other signs of organizing pneumonia |
|
Indeterminate appearance |
Non specific imaging features of COVID 19 pneumonia |
Absence of typical features AND Presence of Multifocal diffuse perihilar or unilateral GGO with or without consolidation lacking a specific distribution and are non rounded or non peripheral Few very small GGO with a non rounded and non peripheral distribution |
|
Atypical Features |
Uncommonly or not reported features of COVID-19 pneumonia |
Absence of typical features or indeterminate features AND Presence of Isolated lobar or segmental consolidation without GGO Discrete small nodules (centrilobar, tree in bud) Lung cavitation Smooth interlobular septal thickening with pleural effusion |
|
Negative for pneumonia |
No features of pnuemonia |
No CT features to suggest pneumonia |
GGO - ground glass opacities
Reference: Simpson S, Kay FY, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt D. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiology: Cardiothoracic Imaging, in press. https://doi.org/10.1148/ryct.2020200152
Typical Features for Pulmonary Involvement of COVID-19
Obligatory Features
Ground-glass opacities, with or without consolidations, in lung regions close to visceral pleural surfaces, including the fissures (subpleural sparing is allowed) and multifocal bilateral distribution
Confirmatory Patterns
Ground-glass regions
Unsharp demarcation, (half ) rounded shape
Sharp demarcation, outlining the shape of multiple adjacent secondary pulmonary lobules
Crazy paving
Patterns compatible with organizing pneumonia
Thickened vessels within parenchymal abnormalities found in all confirmatory patterns
|
Overview of CO-RADS Categories and the Corresponding Level of Suspicion for | ||
|
Pulmonary I |
nvolvement in COVID-19 | |
|
CO-RADS Category |
Level of Suspicion for Pulmonary Involvement of COVID-19 |
Summary |
|
0 |
Not interpretable |
Scan technically insufficient for assigning a score |
|
1 |
Very low |
Normal or noninfectious |
|
2 |
Low |
Typical for other infection but not COVID-19 |
|
3 |
Equivocal/unsure |
Features compatible with COVID-19 but also other diseases |
|
4 |
High |
Suspicious for COVID-19 |
|
5 |
Very high |
Typical for COVID-19 |
|
6 |
Proven |
RT-PCR positive for SARS-CoV-2 |
|
Note.—CO-RADS = COVID-19 Reporting and Data System, COVID-19 = coronavirus disease 2019, RT-PCR = reverse transcription polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. | ||
Reference: Prakap M, Everdingen WV, VEllinga TR et al, for the COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19—Definition and Evaluation. Radiology 2020; 296:E97-E104 • https://doi.org/10.1148/radiol.2020201473
Treatment
Patients categorized to A, B, C must be further risk stratified into mild, moderate and severe.
AVOID using NSAIDs other than paracetamol unless absolutely necessary.
AVOID using nebulized drugs to avoid aerosolization of virus, use MDI instead. Oseltamivir should be initiated in all symptomatic patients with influenza like illness till RTPCR/Antigen test result is obtained.
In patients with COVID-19 pneumonia, secondary bacterial or viral infection is uncommon. Initiation/continuation of antibiotics solely due to COVID-19 is not indicated. Extended duration of fever is typical in COVID-19 patients. Based on literature to date, no unique association between specific pathogens, such as MRSA or Pseudomonas, has been made with COVID-19. Antibiotic selection in case of secondary bacterial pneumonia should be as per institutional antibiogram.
GINA and GOLD guidelines have recommended continuation of inhaled steroids even in patients with COVID-19.
Currently there are no data to support either starting or stopping ACEi /ARBs in any patients with COVID-19. ACEi /ARB may be continued in patients who are already on them. However, if acute kidney injury, hypotension or other contraindication develops, consider stopping them at that time.
If secondary pneumonia is not improving on broad spectrum antibiotics, consider the possibility of CAPA [Covid Associated Pulmonary Aspergillosis] also.
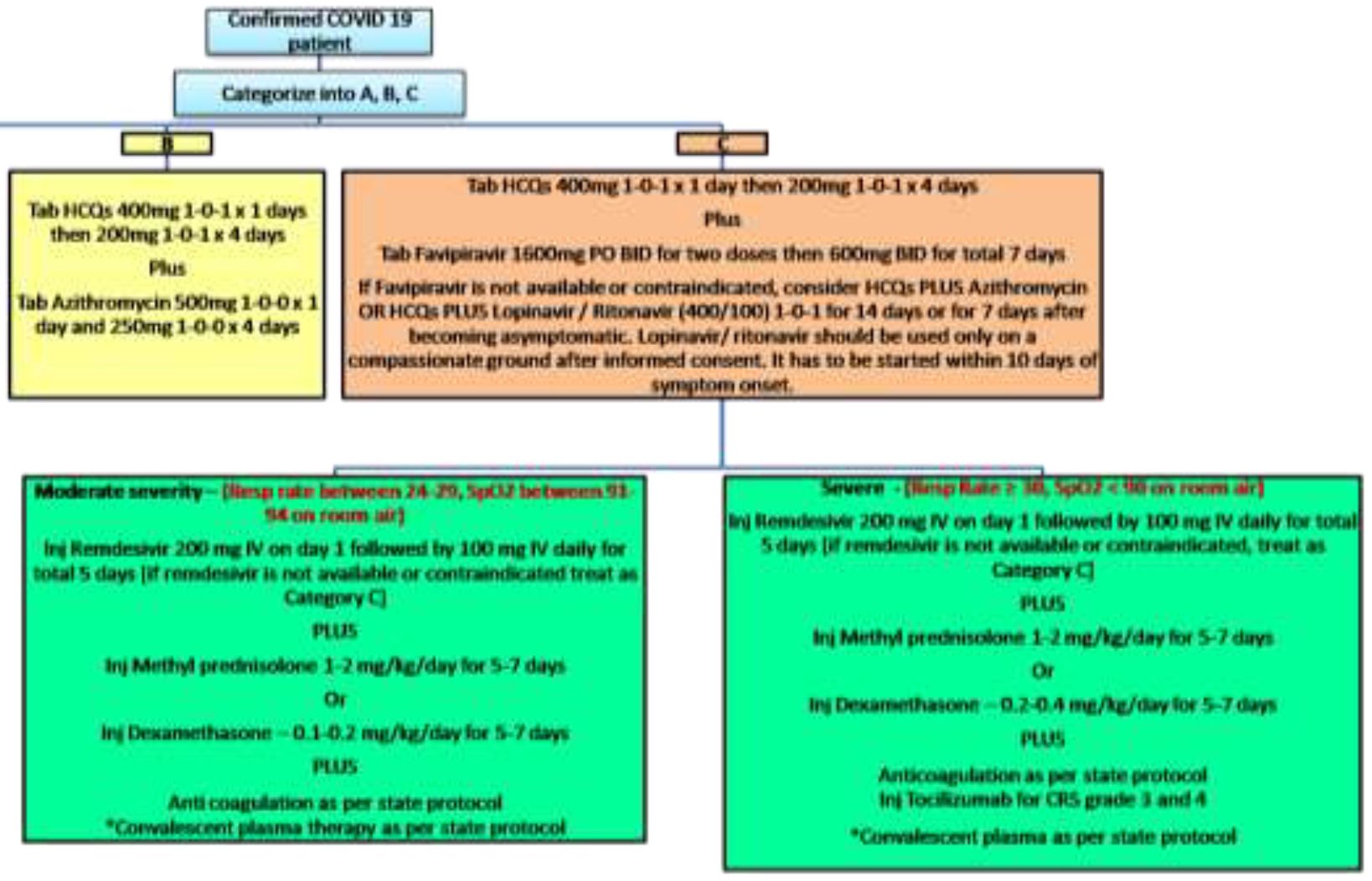
Treatment strategies according to clinical categorization and Risk stratification
<| Category | Treatment | Precautions |
|---|---|---|
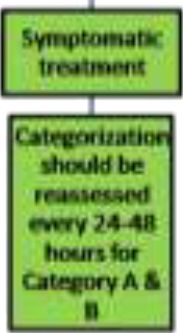
| A | Symptomatic treatment | Categorization should be reassessed every 28-48 hours for Category A. |
| B | Tab HCQs 400mg 1-0-1 x 1 day, then 200 1-0-1 x 4 days* (Children : 6.5mg/kg/ dose PO BD day 1 followed by 3.25mg/kg/dose PO BD X 4 days) Plus Tab Azithromycin 500mg 1-0-0 x 1 day and 250mg 1-0-0 x 4 days Children: 10 mg/kg (max 500mg) day 1, Followed by 5mg/kg/day on days 2 to 5. Tab Oseltamivir 75mg 1-0-1 in all symptomatic patients with influenza like illness until PCR report. Children : 3mg/kg/dose BD Dose adjustment for those with renal insufficiency |
Contraindications to HCQS QTc > 500msec Porphyria Myasthenia gravis Retinal pathology Epilepsy Pregnancy is NOT a contraindication If Baseline QT is prolonged – frequent ECG monitoring is required |
| C | • Tab HCQs 400mg 1-0-1 x 1 day, then 200mg 1-0-1 x 4 days Children : 6.5mg/kg/ dose PO BD day 1 followed by 3.25mg/kg/dose PO BD X 4 days PLUS • Tab Favipiravir 1800mg PO BID for two doses then 800mg BID for total 7 to 10 days • If Favipiravir is not available or contraindicated, consider HCQs PLUS Azithromycin OR HCQs PLUS Tab Lopinavir / Ritonavir (400/100) 1-0-1 for 14 days or for 7 days after becoming asymptomatic. Children ‣ 14 days to 6 months : 16mg/kg (based on lopinavir component) PO BD ‣ < 15kg : 12 mg/kg PO ( based on lopinavir component BD ) ‣ 15-25 kg: 200 mg-50 mg PO BD ‣ 26-35 kg: 300 mg-75 mg PO BD ‣ >35 kg: 400 mg-100 mg PO BD Lopinavir/ritonavir should be used only on a compassionate ground after informed consent. It has to be started within 10 days of symptom onset. |
For chloroquine and derivatives as discussed above For Lopinavir-ritonavir Assess for drug-drug interactions (including with calcineurin inhibitors) before starting. Gastrointestinal intolerance maybe seen Monitor liver function tests while on therapy. Discontinue these agents upon discharge regardless of duration, unless previously used as Maintenance medications for another indication. |
| C – moderate severity (Resp rate between 24-29, SpO2 between 91-94 on room air) | • Inj Remdesivir 200 mg IV on day 1 followed by 100 mg IV daily for 5 days [ If not available treat as Cat C] PLUS < > • Inj Methyl prednisolone 0.5-1 mg/kg/day for 5-7 days Or • Inj Dexamethasone – 0.2-0.4 mg/kg/day for 5-7 days • Convalescent plasma therapy as per state protocol Anti coagulation as per state protocol |
Favipiravir can lead to teratogenicity, transaminitis, neutropenia and dose dependent hyperuricemia. Prior to using favipiravir or remdesivir, pregnancy has to be ruled out in all females in reproductive age group. Favipiravir should not be used in pregnant and lactating females. Favipiravir should be stopped if SGPT >5 times upper limit of normal or if creatinine clearance is <30ml/min/m2 or if there is doubling of creatinine from baseline without an alternative explanation.
*If HCQs is not available Tab Chloroquine base 600 mg (10mg/kg) at diagnosis and 300mg (5 mg/kg) 12 h later, followed by 300 mg (5 mg/kg) BD up to Day 5 [Usually Itablet of chloroquine has 150 mg base]
In Pregnant patients with category C since remdesivir and favipiravir are contraindicated consider using either HCQs PLUS azithromycin OR HCQs PLUS Lopinavir / ritonavir
Zinc sulphate 50mg BD may be added to patients on HCQs / chloroquine
|
A |
Mild sore throat / cough / rhinitis /diarrhea |
|
Fever and/or severe sore throat / cough OR Category-A with any one of the following | |
|
B |
Lung/ heart / liver/ kidney / neurological disease/ Hypertension/haematological disorders/ uncontrolled diabetes/ cancer /HIV- AIDS On long term steroids Pregnant lady Age -more than 60 years. |
|
C |
Breathlessness, chest pain, drowsiness, fall in blood pressure, haemoptysis, cyanosis [red flag signs] Children with ILI (influenza like illness) with redflag signs (Somnolence, high/persistent fever, inability to feed well, convulsions, dyspnoea /respiratory distress, etc). Worsening of underlying chronic conditions. |
Contraindications to chloroquine /HCQS
QTc > 500msec, Porphyria, Myasthenia gravis, Retinal pathology, Epilepsy. Pregnancy is NOT a contraindication. If Baseline QT is prolonged -Monitor ECG daily
Favipiravir can lead to teratogenicity, transaminitis, neutropenia and dose dependent hyperuricemia. Prior to using favipiravir or remdesivir, pregnancy has to be ruled out in all females in reproductive age group. Favipiravir should not be used in pregnant and lactating females. Favipiravir should be stopped if SGPT >5 times upper limit of normal or if creatinine clearance is <30ml/min/m2 or if there is doubling of creatinine from baseline without an alternative explanation.
Remdesivir is contraindicated in
• AST/ALT > 5 times Upper limit of normal (ULN)[AST/ALT has to be monitored daily]
• Severe renal impairment (i.e., eGFR < 30ml/min/m2 or need for hemodialysis)
• Pregnancy or lactating females
In Children: HCQs 6.5mg/kg/ dose BD, day 1 followed by 3.25mg/kg/dose PO BD X 4 days
Lopinavir/Ritonavir (based on lopinavir component): 14 days to 6months : 16mg/kg PO BD, < 15kg : 12 mg/kg PO, 15-25 kg: 200 mg-50 mg PO BD, 26-35 kg: 300 mg-75 mg PO BD, >35 kg: 400 mg-100 mg PO BD
Zinc sulphate 50mg BD may be added to patients on HCQs/ Chloroquine
Criteria for using Tocilizumab
|
Criteria for using Tocilizumab |
Tocilizumab Regimen |
|
Patient should meet ALL the following criteria:
|
8 mg/kg IV (Maximum dose 800 mg] may be repeated once after 12 hours if no clinical improvement. Toxicities/Monitoring Parameters
Cautions
|
5. Cytokine release syndrome [CRS]
|
Grade |
Clinical Assessment |
Treatment |
|
Grade 1 |
Mild reaction: low grade fever, No oxygen requirement or need for IVF |
No treatment |
|
Grade 2 |
Moderate reaction : -High grade fever ( > 103F), need for IVF (not hypotension), mild oxygen requirement (<6L/min) -Grade 2 AKI -Grade 3 LFT (Raised liver enzymes and S. Bilirubin > 2.5gm/dl) |
Send for serum IL-6, If not available , use CRP as a surrogate marker |
|
Grade 3 |
Severe reaction : -Rapidly worsening respiratory status with radiographic infiltrates and spo2 _93% in room air or on supplemental oxygen (> 6L/min, high flow, BiPAP, CPAP)
-Grade 3 AKI, -IVF for resuscitation ,
-low dose vasopressor (Noradrenaline _ 0.5mcg/kg/min or Adrenaline _ 0.3mcg/kg/min) |
Send for serum IL-6 or CRP, Ferritin Consider tocilizumab if there is no response to steroids >18 years : 8mg/kg IV ( max 400mg) _ 18 years _ 30kg : 12mg/kg IV over 60 minutes >30kg : 8mg/kg (max 800mg) IV over 60minutes if no effect can repeat x 2 more doses Q8H apart; |
|
Grade 4 |
Life threatening multi organ dysfunction, hypoxia requiring mechanical ventilation, hypotension requiring high dose vasopressors |
Send for serum IL-6 or CRP; consider tocilizumab as in Grade 3 if there is no response to steroids. |
(Adapted and modified from the Penn CRS criteria and MGH)
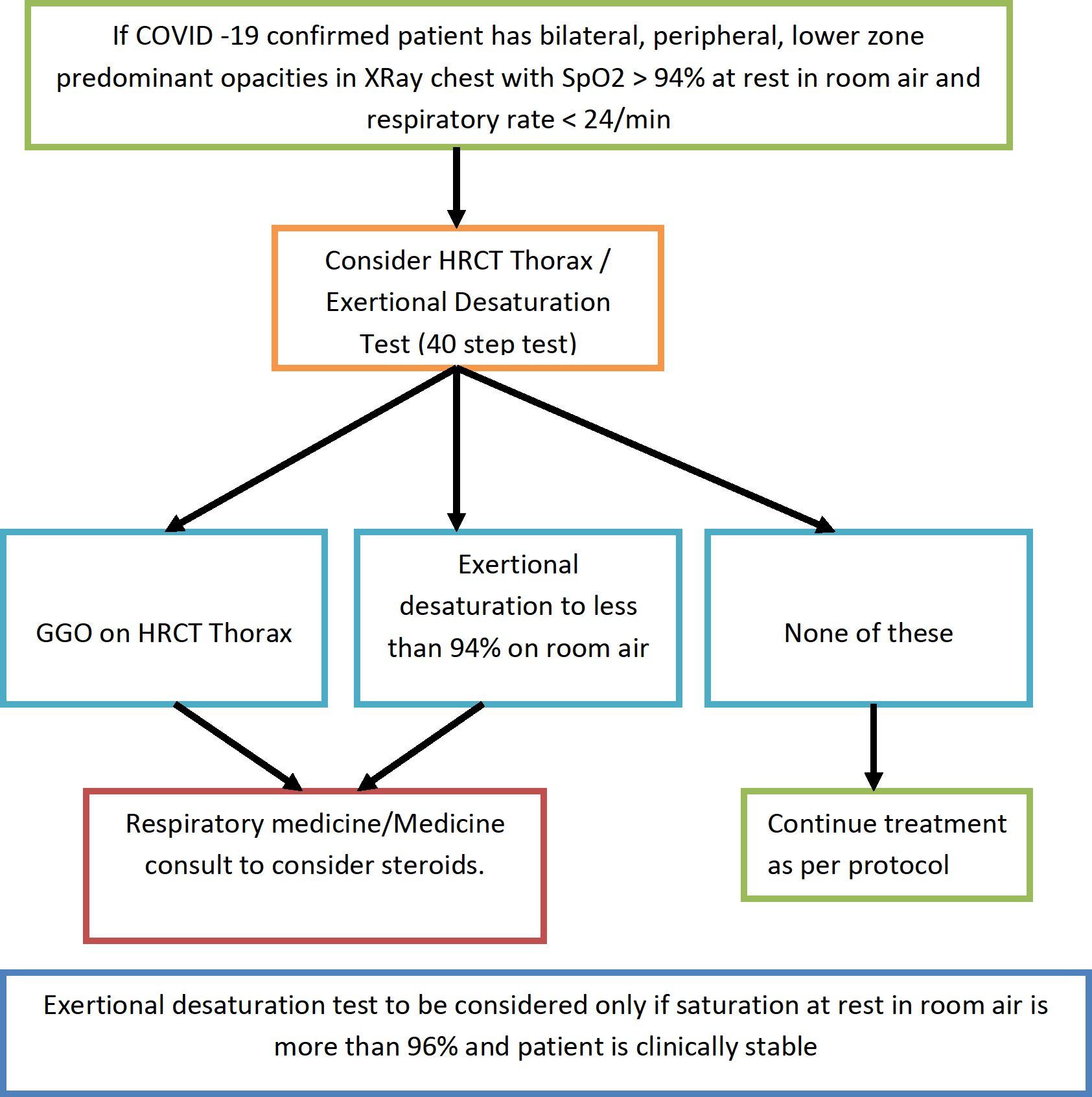
Algorithmic approach to patients with exertional desaturation / radiological evidence of interstitial pneumonia without hypoxia at rest
6. Anticoagulation
PATHOGENESIS OF COVID - 19 AND THE NEED FOR ANTICOAGULATION
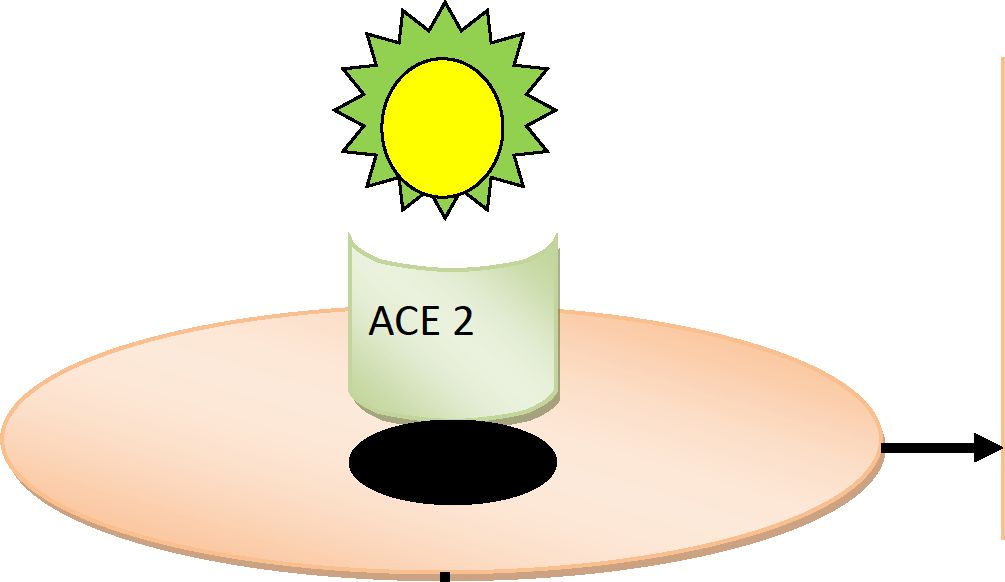
Host cell present in Vessels
Lungs
Heart
Kidneys
GI tract Biliary tract
Excessive immune response - cytokine storm - ^levels of IL=1, IL- 2, IL-6, IF - y, TNF - a, G-CSF and others
Local and systemic inflammatory response - SIRS
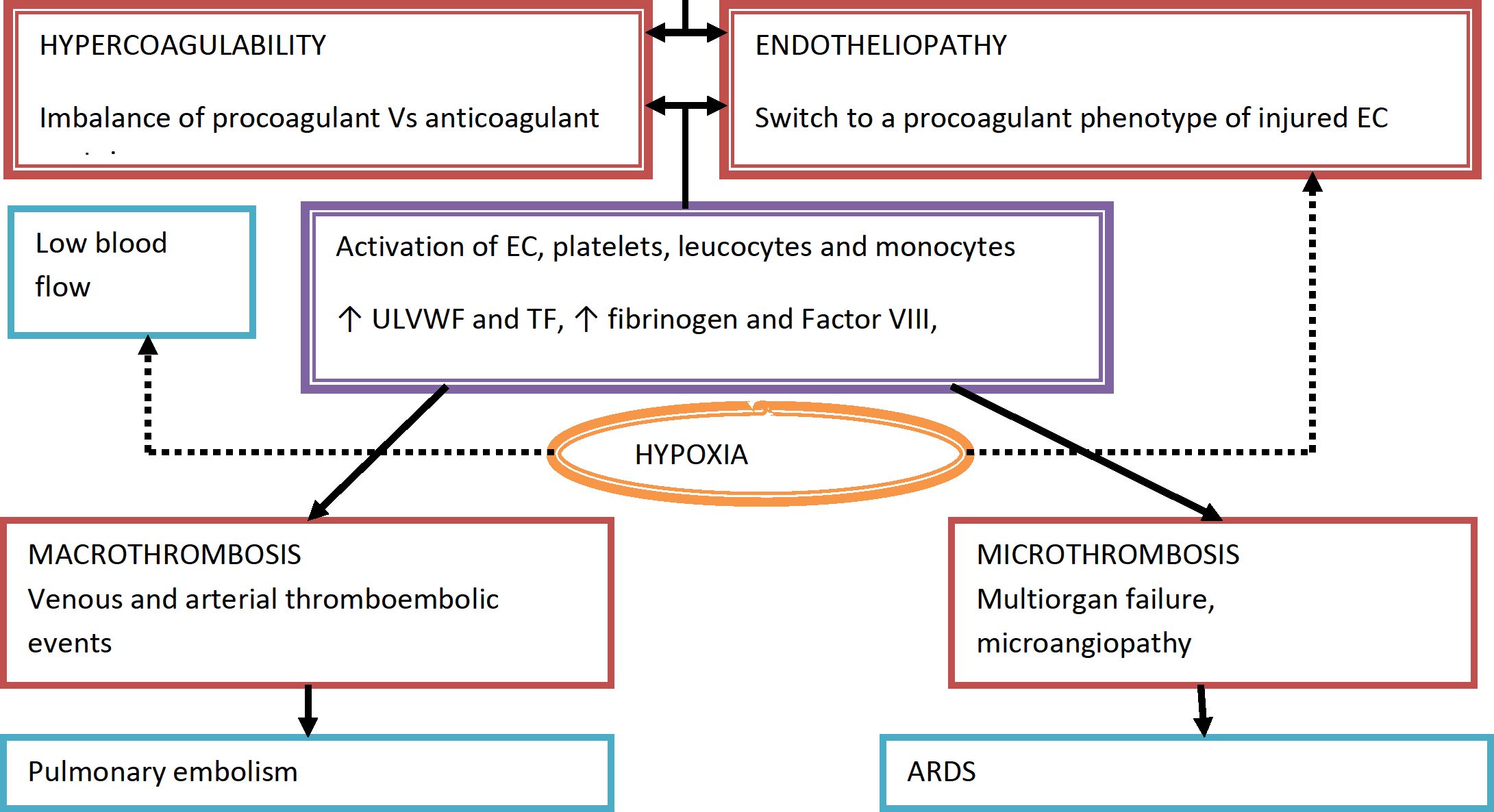
Moderate illness: individuals who have evidence of lower respiratory illness by clinical assessment or imaging and a saturation of oxygen (SpO2 < 94%) on room air at sea level
Serious illness: Individuals who have respiratory frequency >30 breaths/min, SpO2 <90% on room air at sea level,
PaO2/FIO2 < 300 or lung infiltrates > 50%
For COVID positive on Therapeutic anticoagulation during hospitalization, Tab Apixaban 5mg BD to be continued for minimum of 2 weeks after discharge.
To reduce dose to 2.5mg BD if Age > 80years or weight < 60kg
|
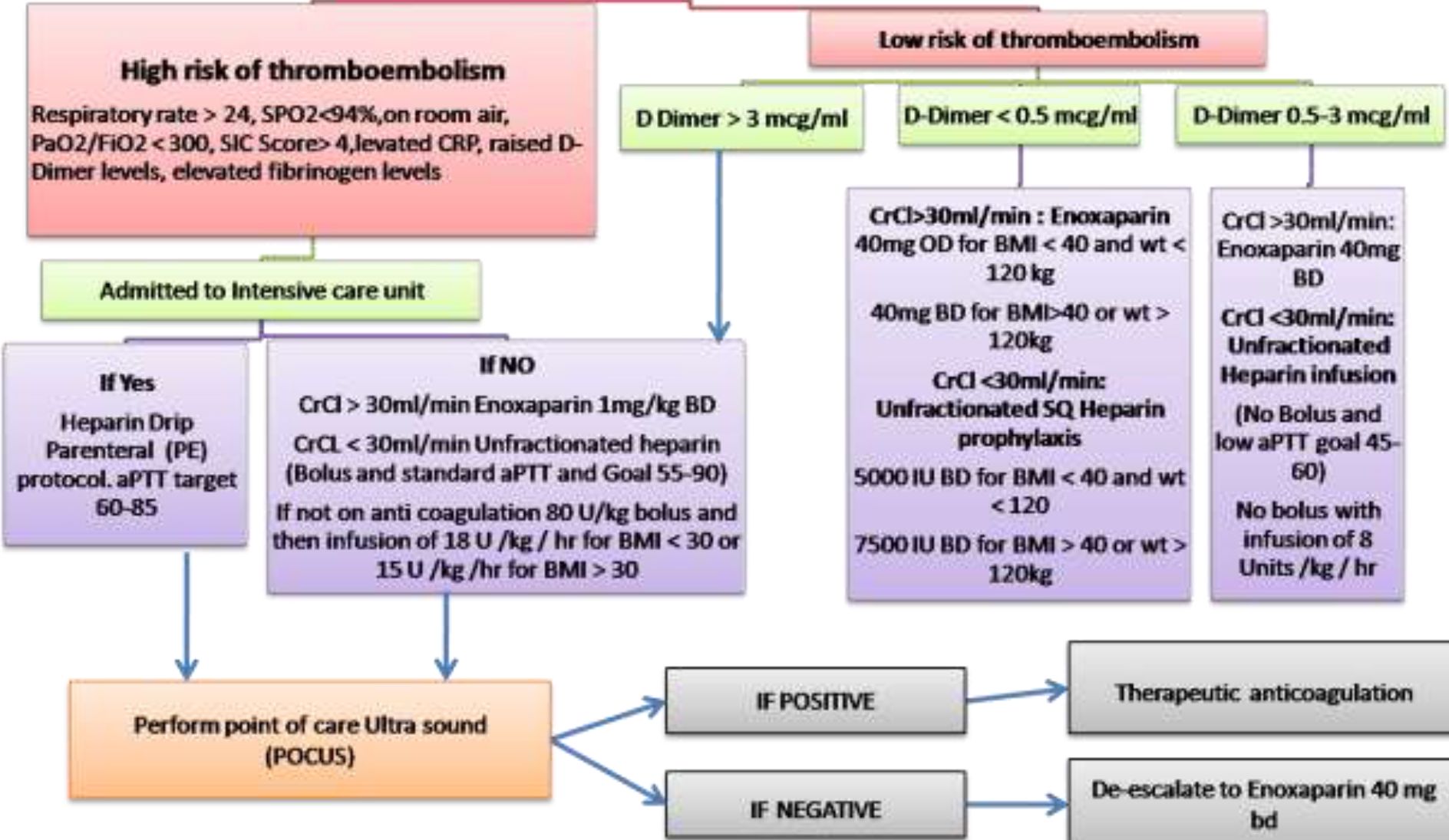
SIC score | ||||
|
Category |
Parameter |
0 point |
1 point |
2 points |
|
P rothrombin time |
PT-INR |
<1.2 |
>1.2 |
>1.4 |
|
Coagulation |
Total Platelet Count ( x 10A9/ml) |
>150 |
<150 |
<100 |
|
Total SOFA |
SOFA four items |
0 |
1 |
>2 |
Hold anticoagulation if platelet count < 25,000/ml or evidence of current or recent bleeding
If taking anticoagulation at home for any previous indication, may switch to enoxaparin or heparin as per algorithm for the duration of hospitalization unless contraindicated
When to suspect Pulmonary Embolism:
This is a challenge given the inherent hypoxia and altered coagulation profile observed in COVID-19 infected patients.
Consider PE in the case of:
-
a. Marked increase/rising D-dimer from baseline AND
-
b. Acute worsening of oxygenation, blood pressure, tachycardia with imaging findings NOT consistent with worsening COVID-19 Pneumonia.
Rationale for early anti coagulation
-
• Pathophysiology of COVID - 19 associated respiratory disease is consistent with pulmonary vascular thromboemboli with increased dead space ventilation
-
• Autopsy studies have demonstrated venous thrombo embolism in deceased Corona patients
-
• Early anticoagulation is necessary to prevent propagation of micro thrombi at disease presentation
-
• Early anticoagulation may be associated with decreased mortality
Rationale for choice of Anticoagulant
-
• Heparin binds tightly to COVID - 19 spike protein
-
• Heparin also downregulate IL-6 and directly dampen immune activation
References
-
1. Bassam Atallah, Saad I Mallah, Wael AlMahmeed, Anticoagulation in COVID-19, European Heart Journal - Cardiovascular Pharmacotherapy, pvaa036
-
2. Massachusets General Hospital Hematology Recommendations and Dosing Guidelines during COVID-19
-
3. Mount Sinai anticoagulation algorithm
-
4. Joly, B.S., Siguret, V. & Veyradier, A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med (2020)
7. Compassionate use of convalescent plasma for treatment of patients with moderate to severe COVID 19 infection
Compassionate use of convalescent plasma may be considered in
-
1. Laboratory confirmed diagnosis of infection with SARS CoV 2
-
2. COVID - 19 with moderate/severe disease
-
3. Informed consent provided by the patient or relative
-
4. Emergency approval from state medical board
Moderate COVID 19
Respiratory rate 24-29/min
SpO2 < 94% on room air
Severe COVID infection
Respiratory rate > 30/min
SpO2 < 90% on room air
Ratio of partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300 Lung infiltrates > 50% within 24-48 hours
Exclusion criteria
-
1. Lack of consent
-
2. Known hypersensitivity to blood products
-
3. Known IgA deficiency or immunoglobulin allergy.
Eligibility of Donor
-
• > 18 years of age
-
• Males or female donors of weight > 55kg
-
• Prior diagnosis of COVID - 19 documented by a laboratory test (RT-PCR) with symptomatic disease with at least fever and cough OR preferably plasma IgG titre [against S-protein] should be above 1:640.
-
• Complete resolution of symptoms at least 28 days to donation
-
• Further technicalities with regard to donor eligibility will be decided by transfusion medicine departments of designated COVID-19 treatment facilities.
OR
-
• In addition donor eligibility criteria for whole blood donation will be followed in accordance to the drugs and cosmetics Act 1940 and rules 1945 therein ( as amended till March 2020)
SOP for convalescent plasma administration under compassionate grounds
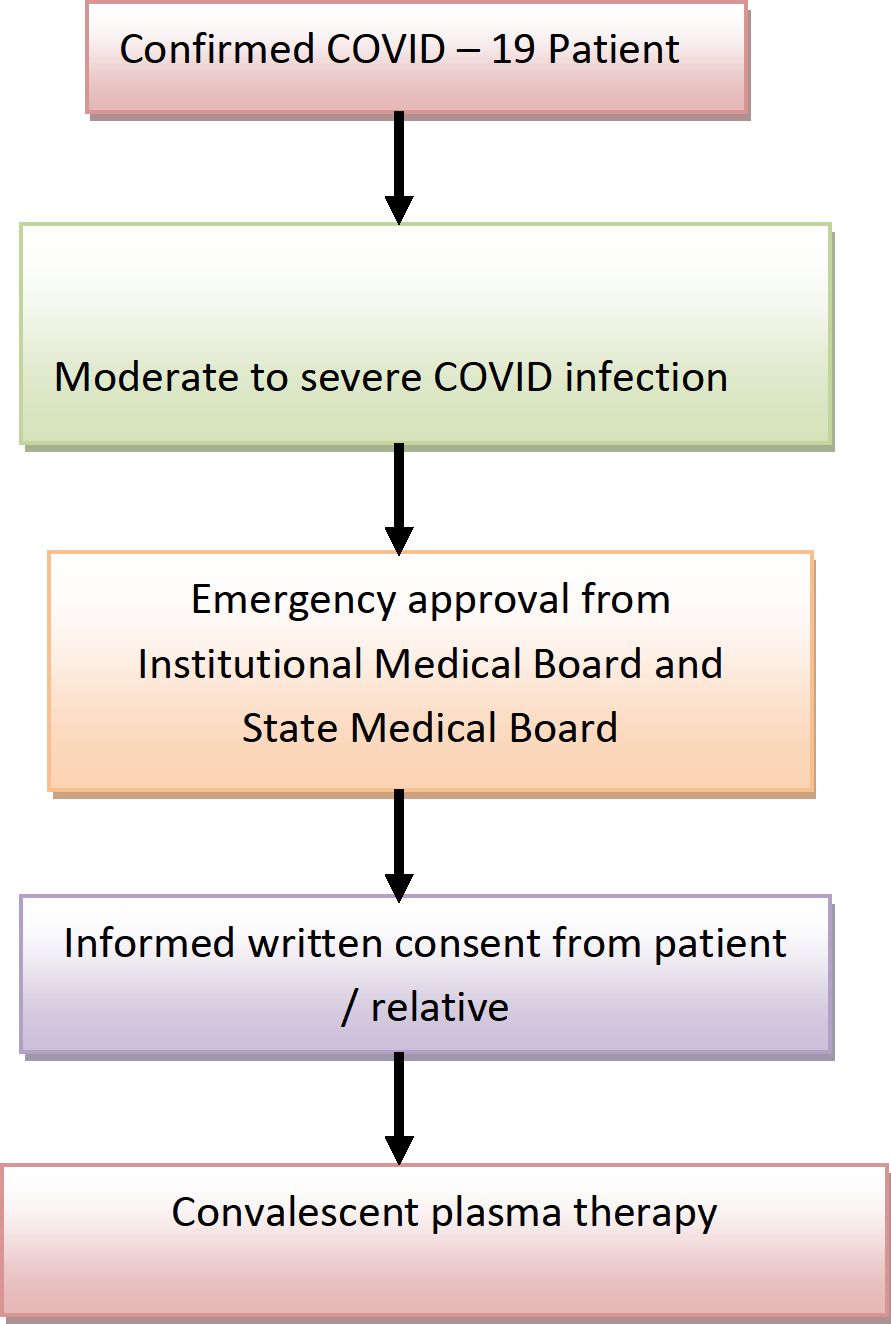
Dose of convalescent plasma
ABO compatible plasma transfusion of 200ml will be followed by one additional dose of 200ml at 24 hours interval unless contraindicated. Hence the cumulative dose of convalescent plasma for each patient will be 400ml.The second plasma unit will preferably be from a different donor depending on availability of another ABO compatible plasma unit or else plasma unit from the same donor will be issued.
Reference
-
1. Recommendation for investigational use of COVID -19 convalescent plasma - US FDA
-
2. Clinical management protocol :COVID-19: Government of India Version 3
-
3. ICMR: PLACID trial protocol
8. Chemoprophylaxis
The National Task force for COVID-19 constituted by ICMR recommends the use of hydroxychloro quine for prophylaxis of SARS-CoV -2 infection for high risk population.
Asymptomatic healthcare workers involved in the care of suspected or confirmed cases of COVID-19.
Asymptomatic household contacts of laboratory confirmed cases
DOSE
Asymptomatic healthcare workers involved in the care of suspected or confirmed cases of COVID-19: 400 mg twice a day on Day 1, followed by 400 mg once weekly for next 7 weeks : to be taken with meals.
Asymptomatic household contacts of laboratory confirmed cases: 400 mg twice day on Day 1, followed by 400 mg once weekly for next 3 weeks, to be taken with meals.
Exclusion/contraindication
Drug is not recommended for prophylaxis in children under 15 years of age.
Drug is contraindicated in persons with retinopathy, hypersensitivity to HCQS or 4-aminoquinoline compounds
Hydroxychloroquine prophylaxis is to be taken ONLY as per prescription of a physician and baseline QTc interval should be calculated for all persons prior to administering the drug
9. REMDESIVIR
Remdesivir is an intravenous (IV) investigational nucleotide prodrug of an adenosine analog. Remdesivir binds to the viral RNA-dependent RNA polymerase, inhibiting viral replication through premature termination of RNA transcription. It has demonstrated in vitro activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 In a rhesus macaque model of SARS-CoV-2 infection, remdesivir treatment was initiated soon after inoculation; remdesivir-treated animals had lower virus levels in the lungs and less lung damage than the control animals.2
Remdesivir has been studied in several clinical trials for the treatment of COVID-19. The recommendations for use of Remdesivir is based on the results of these trials
Recommendation for Prioritizing Limited Supplies of Remdesivir
-
• Since supplies are limited, remdesivir should be prioritized for use in hospitalized patients with COVID-19 who require supplemental oxygen but who are not on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO)
Recommendation for Patients with COVID-19 Who Are on Supplemental Oxygen but Who Do Not Require High-Flow Oxygen, Noninvasive or Invasive Mechanical Ventilation, or ECMO
-
• Use remdesivir for 5 days or until hospital discharge, whichever comes first .
If a patient who is on supplemental oxygen while receiving remdesivir progresses to requiring high-flow oxygen, noninvasive or invasive mechanical ventilation, or ECMO, the course of remdesivir should be completed.
Recommendation for Patients with COVID-19 Who Require High-Flow Oxygen, Noninvasive Ventilation, Mechanical Ventilation, or ECMO
-
• There is uncertainty regarding whether starting remdesivir confers clinical benefit in these groups of patients, so a recommendation either for or against starting remdesivir.cannot be made based on the available evidence till now.
In a randomized clinical trial, there was no observed difference between the remdesivir and placebo groups in time to recovery or mortality rate in these subgroups. However, because the trial was not powered to detect differences in outcomes in these subgroups, there is uncertainty as to the effect of remdesivir on the course of COVID-19 in these patients.
Duration of Therapy for Patients Who Have Not Shown Clinical Improvement After 5 Days of Therapy
-
• There are insufficient data on the optimal duration of remdesivir therapy for patients with COVID-19 who have not shown clinical improvement after 5 days of therapy. In this group, some experts extend the total remdesivir treatment duration to up to 10 days .

Rationale
The recommendations for remdesivir are largely based on data from a multinational, randomized, placebo-controlled trial (the Adaptive COVID-19 Treatment Trial [ACTT]). This trial included 1,063 hospitalized patients with COVID-19 and evidence of lower respiratory tract infection who received IV remdesivir or placebo for 10 days (or until hospital discharge, whichever came first).
Participants who received remdesivir had a shorter time to clinical recovery than those who received placebo (median recovery time of 11 days vs. 15 days, respectively). In the preliminary subgroup analyses of ACTT, there was no observed benefit for remdesivir in people with COVID-19 who did not require oxygen supplementation; however, the number of people in this category was relatively small. Remdesivir is being evaluated in another clinical trial for the treatment of patients with moderate COVID-19; complete data from this trial are expected soon.
The preliminary analysis also reported that the patients with the clearest evidence of clinical benefit from starting remdesivir were those who required supplemental oxygen but who did not require high-flow oxygen, noninvasive or mechanical ventilation, or ECMO at baseline (n = 421). In this subgroup, those who received remdesivir had a shorter time to recovery than those who received placebo (recovery rate ratio 1.47; 95% confidence interval [CI], 1.171.84); in a post-hoc analysis of deaths by Day 14, remdesivir appeared to confer a survival benefit (hazard ratio [HR] for death 0.22; 95% CI, 0.08-0.58).
In patients who required high-flow oxygen or noninvasive ventilation at baseline (n = 197), there was no observed difference in time to recovery between the remdesivir and placebo groups (recovery rate ratio 1.20; 95% CI, 0.79-1.81). In the post-hoc analysis of deaths by Day 14, there was no evidence that remdesivir had an impact on the mortality rate in this subgroup (HR 1.12; 95% CI, 0.53-2.38).
In participants who were on mechanical ventilation or ECMO at baseline (n = 272), there was no observed difference in time to recovery between the remdesivir and placebo groups (recovery rate ratio 0.95; 95% CI, 0.64-1.42). In the post-hoc analysis of deaths by Day 14, there was no evidence that remdesivir had an impact on the mortality rate in this subgroup (HR 1.06; 95% CI, 0.59-1.92).
A review of the final data set, which included 28-day mortality, showed that this data set was consistent with the published preliminary data (unpublished data, based on communication from the ACTT study team to the Panel).
For patients with COVID-19 who required high-flow oxygen, noninvasive ventilation, mechanical ventilation, or ECMO, there was no observed difference between the remdesivir and placebo groups in time to recovery or mortality rate. However, because the trial was not powered to detect differences in outcomes within these subgroups, there is uncertainty as to whether starting remdesivir confers clinical benefit in these patients. For this reason, a recommendation cannot be made either for or against starting remdesivir in these patients. Because the supply of remdesivir is limited, the drug should be prioritized for use in those in whom efficacy has been demonstrated (i.e., in hospitalized patients who require supplemental oxygen but who are not on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or ECMO).
Data from a multinational, open-label trial of hospitalized patients with severe COVID-19 showed that remdesivir treatment for 5 or 10 days had similar clinical benefit.The optimal duration of therapy for patients who do not improve after 5 days of receiving remdesivir is unclear. In the absence of data, some experts consider extending the total treatment duration of remdesivir to up to 10 days in patients who do not improve after 5 days of remdesivir.
Monitoring, Adverse Effects, and Drug-Drug Interactions
Remdesivir can cause gastrointestinal symptoms (e.g., nausea, vomiting), elevated transaminase levels, and an increase in prothrombin time (without a change in the international normalized ratio).
Clinical drug-drug interaction studies of remdesivir have not been conducted. Remdesivir levels are unlikely to be substantially altered by cytochrome P450 (CYP) 2C8, CYP2D6, or CYP3A4 enzymes, or by P-glycoprotein (P-gp) or organic anion-transporting polypeptide (OATP) drug transporters.
Remdesivir may be administered with weak to moderate inducers or with strong inhibitors of CYP450, OATP, or P-gp. Strong induction may modestly reduce remdesivir levels. The clinical relevance of lower remdesivir levels is unknown. The the use of remdesivir with strong inducers (e.g., rifampin) is not recommended.
Minimal to no reduction in remdesivir exposure is expected when remdesivir is coadministered with dexamethasone. Chloroquine or hydroxychloroquine may decrease the antiviral activity of remdesivir; coadministration of these drugs is not recommended.
Because the remdesivir formulation contains renally cleared sulfobutylether-beta-cyclodextrin sodium, it is contraindicated in patients with creatinine clearance _30ml/min/m2
Considerations in Pregnancy
-
• Use remdesivir in pregnant patients only when the potential benefit justifies the potential risk to the mother and the fetus. It should be considered in pregnancy only on compassionate grounds after getting consent from the state medical board.
-
• The safety and effectiveness of remdesivir for treatment of COVID-19 have not been evaluated in pregnant patients.
-
• Remdesivir is available through the Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for adults and children and through compassionate use programs for pregnant women and children with COVID-19.
Considerations in Children
-
• The safety and effectiveness of remdesivir for treatment of COVID-19 have not been evaluated in pediatric patients.
-
• Remdesivir is available through an FDA EUA for adults and children and through compassionate use programs for children with COVID-19. A clinical trial is currently evaluating the pharmacokinetics of remdesivir in children (ClinicalTrials.gov identifier NCT04431453).
10. Available evidence on the use of Tocilizumab in COVID-19
Tocilizumab is a recombinant humanized monoclonal antibody against IL-6 receptor
Rationale for use of Tocilizumab in COVID-19
Pro-inflammatory cytokine levels are elevated in COVID-19 infection. Predictors of mortality from a retrospective, multicentre study of 150 confirmed COVID-19 cases in Wuhan, China included elevated ferritin and IL-6. This suggests that virus induced hyper inflammation is contributing to the mortality1, 2.
Tocilizumab has been found useful in severe or life threatening cases of cytokine release syndrome (CRS) due to chimeric antigen receptor-T cell therapy. However there are no randomized control trials that compared Tocilizumab versus steroids for CRS 3.
Dose recommended for CRS: >18 years: 8mg/kg IV (400mg),
< 18 years:
< 30kg: 12mg/kg IV over 60 minutes
>30kg: 8mg/kg (max 800mg) IV over 60minutes
The total tocilizumab dose should not exceed 800 mg4.
If no effect can repeat x 1 more dose after 8 to 12 hours
Can be given as an intravenous infusion in normal saline over 1 hour.
Dose modification of Tocilizumab in case of liver enzyme derangement
|
Liver enzymes 1 -3 times upper limit of normal |
For patients receiving intravenous Tocilizumab, reduce dose to 4 mg per kg or hold the drug until ALT or AST have normalized |
|
Liver enzymes 3-5 times upper limit of normal |
Hold Tocilizumab dosing until less than three times upper linit of normal and follow recommendations above for greater than 1 to three times upper limit of normal For persistent increases greater than three times upper limit of normal, discontinue |
|
Liver enzymes > 5 times upper limit of normal |
Discontinue the drug |
Drug dosing based on absolute neutrophil count and platelet count
|
Lab Parameter (cells/mm3) |
Recommendation |
|
Anc >1000 |
Maintain drug dose |
|
ANC 500- 1000 |
Hold Tocilizumab dosing When ANC greater than 1000 cells per mm3 : • For patients receiving intravenous drug, resume Tocilizumab at 4 mg per kg and increase to 8 mg per kg as clinically appropriate • For patients receiving subcutaneous tocilizumab, resume drug at every other week and increase frequency to every week as clinically appropriate |
|
ANC less than 500 |
Discontinue the drug |
|
Platelet count 50,000 - 1,00,000 |
Hold Tocilizumab dosing When platelet count is greater than 100,000 cells per mm3 : • For patients receiving intravenous drug, resume Tocilizumab at 4 mg per kg and increase to 8 mg per kg as clinically appropriate . |
|
Platelet count < 50,000 |
Discontinue Tocilizumab |
.Guidelines and recommendations:
1) Recommendations for COVID-19 clinical management, National Institute for the Infectious Diseases, Italy:
Tocilizumab: 8 mg/kg (maximum 800 mg/dose), single dose intravenously (1-hour infusion); in absence or with poor clinical improvement a second dose should be administered after 812 hours.
Tocilizumab administration should be guided by the presence of 1 or more of following selection criteria: a) PaO2/Fi02 ratio < 300, b)rapid worsening of respiratory gas exchange with or without availability of non-invasive or invasive ventilation c)IL-6 levels >40 pg/ml (if not available, see D-dimer levels >1000 ng/ml.)
Therapeutic schedule: 2 administrations (each 8 mg/kg, maximum 800 mg). Second administration to be started at 8-12 hours from the first one. Repeat PCR and D-dimer (+/-IL-6) after 24 hours from each administration.
2) Massachusetts General Hospital COVID-19 Treatment Guidance:
To be given after establishment of clinical status
Grade 1 - mild reaction
Grade 2 - moderate reaction, fever, need for IVF (not hypotension), mild oxygen requirement
Grade 3 - severe, liver test dysfunction, kidney injury, IVF for resuscitation, low dose vasopressor, supplemental oxygen (high flow, BiPAP, CPAP)
Grade 4 - life threatening, mechanical ventilation, high dose vasopressors
Treatment interventions based on grades:
Grade 1 - no treatment
Grade 2 - send for serum IL-6
Grade 3 - send for serum IL-6; consider Tocilizumab, if no effect can repeat x 2 more doses Q8H apart; if
no response, consider low dose corticosteroids
Grade 4 - send for serum IL-6; consider Tocilizumab as Grade 3; consider corticosteroids
References:
-
1. COVID-19: consider cytokine storm syndromes and immunosuppression - The Lancet
[Internet]. [cited 2020 Mar 21]. Available from:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30628-0/fulltext
-
2. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. - PubMed - NCBI [Internet]. [cited 2020 Mar 21]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32125452
-
3. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. -PubMed - NCBI [Internet]. [cited 2020 Mar 21]. Available from: https://www.ncbi.nlm.nih.gov/pubmed?term=27913530
-
4. ACTEMRA (tocilizumab) injection. Drug monograph
-
5. Xu et al Effective Treatment of Severe COVID-19 Patients with Tocilizumab. China Xiv:202003.00026v1
-
11. GUIDELINES FOR COMPASSIONATE USE OF LOPINAVIR/RITONAVIR
IN SYMPTOMATIC -COVID -19 PATIENTS
Treatment with lopinavir-ritonavir should be restricted to those patients with proven -COVID-19 who present with clinical syndromes of mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis or septic shock.
Patient Eligibility criteria:
Laboratory confirmation of -COVID-19 infection.
Patients with mild Pneumonia, severe pneumonia, ARDS, Sepsis or septic shock, hospitalized due to symptoms related to COVID-19.
Informed consent from patient
Clearance from Institutional Medical Board
Exclusion Criteria:
® Asymptomatic individuals with COVID-19 infection.
® Known allergy or hypersensitivity reaction to Lopinavir / Ritonavir.
® A patient with Hepatic Impairment (ALT over more than five times the normal).
® Use of medications that are contraindicated with Lopinavir / Ritonavir and that cannot be replaced or stopped, It is contraindicated with astemizole, terfenadine, cisapride, ergot derivatives, sildenafil, midazolam, triazolam; lovastatin, simvastatin, pimozide and fluticasone propionate.
® Known HIV infected individual receiving other protease inhibitors containing regimen
® Documented chronic liver disease.
DOSAGE OF LOPINAVIR / RITONAVIR
ADULTS:
Lopinavir / Ritonavir 200mg/50mg - 2 tablets every 12 hours for 14 days or for 7 days after becoming asymptomatic whichever is earlier
For patients unable to take medicines orally, 400mg Lopinavir / 100 mg Ritonavir 5ml suspension every 12 hours for 14 days or for 7 days after becoming asymptomatic whichever is earlier, via a nasogastric tube.
Administer with caution among persons receiving Rifampicin, Ketoconazole, ethylene estradiol.
LABORATORY SAMPLE COLLECTION-(other than investigations for routine clinical monitoring)
► Blood sample every 48 hours — for PT/INR, LFT, RFT and serum amylase (to monitor drug-induced adverse events)
FREQUENCY AND DURATION OF MONITORING:
Patients should be monitored daily until discharge from the hospital by the Institutional Medical Board.
Patient should be discharged based on the State protocol in concurrence with the opinion of Institutional Medical Board.
Adverse events of Lopinavir -ritonavir
The observed adverse effects with lopinavir/ritonavir are
-
1. Acute pancreatitis (defined as having)
-
a. abdominal pain consistent with acute Pancreatitis
-
b. serum amylase at least three times greater than the upper limit of normal)
-
2. Elevation of ALT to more than five-fold upper limit of normal.
-
3. Anaphylaxis
-
4. Bleeding diathesis (INR > 3 without anticoagulant therapy)
-
5. Diarrhoea.
12. Adult critical care guidelines
-
I. CASE DEFINITION -CRITICAL
-
1. Respiratory failure, requiring Mechanical ventilation (PaO2<60 with FiO2>0.5 with or without PaCO2>50mmHg with pH<7.25)
-
2. Shock
-
3. Other organ failure requiring ICU admission
II. SEVERE &CRITICAL CASE: MANAGEMENT 1. Assess:
-
a. General: PR, HR, BP, Respiratory Rate, SpO2, Work of breathing
-
b. SpO2
-
c. Tidal volume generated if on NIV
-
d. Level of consciousness
-
e. Organ function
-
f. System examination
-
g. Screening echo
h.
|
Labs: | |
|
i. |
BRE: Hb, TC, DC,Platelet count |
|
ii. |
URE |
|
iii. |
LFT |
|
iv. |
RFT |
|
v. |
Lactate |
|
vi. |
Blood sugar |
|
vii. |
CRP |
|
viii. |
Procalcitonin |
|
ix. |
Coagulation profile |
|
x. |
Ferritin,LDH |
|
xi. |
ECG |
|
xii. |
hsTrop T/ Trop I |
|
xiii. |
Chest Imaging |
|
xiv. |
NTProBNP |
-
2. Warning Indicators: Increasing work of breathing, progressive decrease of peripheral Absolute lymphocyte count, increasing levels of IL-6/ C-reactive protein, Tissue oxygenation indices decreases, Lactate level increasing progressively, Chest CT/Xray shows obvious progression of lung lesions.
-
3. COVID-19 patients appear to havetwo phenotypes, from the perspective of ICU management (Gattinoni et al, 2020). Management should be optimised for each individual patient as clinically indicated, based on established strategies for the management of ARDS.
L-phenotype
-
a. Typical of early presentation viral pneumonitis
-
b. Hypoxaemia with preserved CO2 clearance (Type 1 respiratory failure)
-
c. LowElastance (i.e. high compliance)
-
d. Low V/Q matching (possibly due to abnormal hypoxic vasoconstriction)
-
e. Low recruitability (poor response to PEEP and prone position ventilation)
-
f. May be able to avoid mechanical ventilation with appropriate oxygen therapy
H phenotype
-
a. Typical of later illness and classic ARDS, including patients who have had prolonged non-invasive ventilation (potential for patient-induced lung injury) and co-existing lung disease or complications
-
b. Hypoxaemia +/- impaired CO2 clearance (Type 1 and/or 2 respiratory failure)
-
c. HighElastance (i.e. low compliance)
-
d. High V/Q matching
-
e. High recruitability (respond to PEEP and prone position ventilation)
-
f. May benefit from protective lung ventilation and usual ARDS therapies.
-
4. Treatment
-
a. General Principle: Bed rest, maintain fluid balance, acid base, oxygen therapy, mechanical ventilation in time, prevent &treat complications of critically ill, treat disease, prevent secondary infections, prevent transmission.
-
b. Antiviral treatment: as per state guidelines
-
c. Oxygen therapy & respiratory support
-
i. PaO2/FiO2 at 200-300
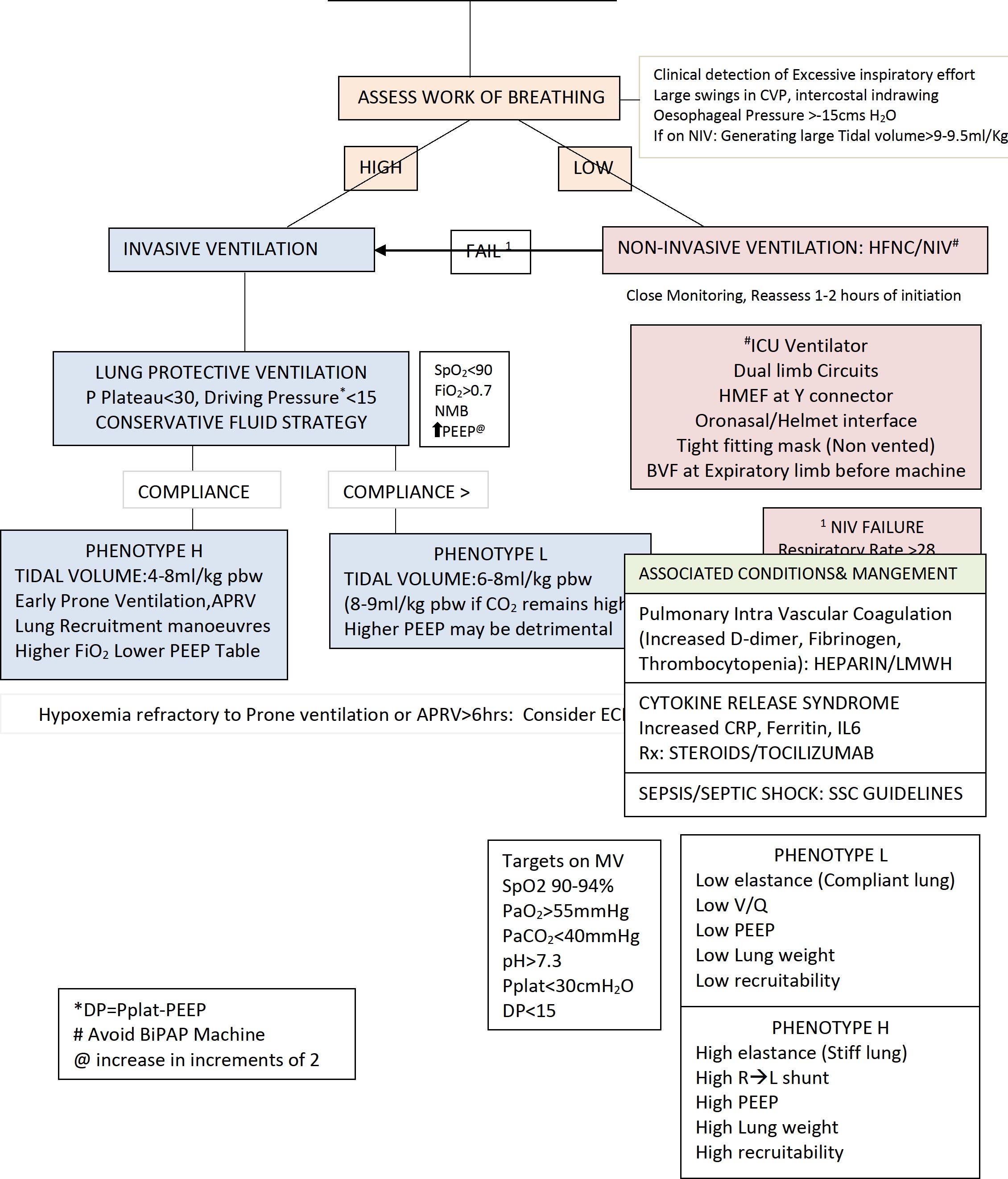
-
1. Nasal cannula /oxygen mask. Assess if respiratory disease/hypoxemia has remitted. If no improvement in 1-2 hours— go to next step/invasive mechanical ventilation
-
2. High flow nasal cannula HFNC: limited recommendation. If used patient ideally in Negative pressure room or single isolation room with good ventilation. More aerosol formation. Maximum for 2 hours. Close observation is needed. If no improvement /response (Respiratory Rate >35/minSpO2<93%, increased work of breathing, accessory muscle use)- consider intubation and mechanical ventilation.
-
ii. PaO2/FiO2 at 150-200
1. Non-invasive ventilation (NIV): Limited use, more aerosols.In Negative pressure room or single isolation room with good ventilationWhen using NIV, double circuit NIV with oronasal/helmet interface, non-vented mask preferred. Use HEPA filter at expiratory limb & HMEF at Y connector. Should not be used for transportation. Failure rate is high, close monitoring required. If no improvement in 12 Hours invasive mechanical ventilation initiated promptly. Monitor for “no improvement” or worsening (Respiratory Rate >35/minSpO2<93%, accessory muscle use, generating large tidal volume>9-9.5ml/kg).
-
iii. PaO2/FiO2<150
1. Invasive mechanical ventilation
-
a. Early appropriate mechanical ventilation
-
b. Lung protective strategy (P plateau <30, Driving Pressure <15 cms H2O). Tidal Volume 4-8 ml/kg predicted body weight (PBW). Permissive hypercapnia may be permitted to reduce volutrauma.The initial tidal volume is 6 mL/kg PBW; tidal volume up to 8 mL/kg PBW is allowed if undesirable side effects occur (e.g. dyssynchrony, pH < 7.15) and also for L phenotype with CO2 retention.
-
c. Ards.net guidelines to be followed especially for H phenotype,
-
d. PEEP: Gradual increase in increments of 2, with hemodynamic monitoring. Other options include: Optimal PEEP via static compliance method/ FiO2/PEEP table in ARDS.net guideline
-
e. Neuro muscular blockade: to be considered in the setting of worsening hypoxia or hypercapnia and in situations where the patient's respiratory drive cannot be managed with sedation alone resulting in ventilator dyssynchrony and lung decruitment.
-
f. Lung recruitment:Although current evidence does not support the routine use of recruitment manoeuvres in non-COVID-19 ARDS, they could be considered in COVID-19 patients on a case by case basis. COVID-19 patients may respond well to these interventions and their application may be appropriate where the patient has not responded to other interventions. They should only be provided by clinicians experienced in undertaking these manoeuvres, dealing with their potential complications and using a closed system. Methods: See below.
-
g. Prone position Ventilation: EARLY prone ventilation may be considered for refractory hypoxemia. Effective in improving hypoxia associated with COVID-19 H phenotype. This should be done in patients with refractory hypoxemia and not improving with standard lung protective ventilation strategy and policies should include suitable PPE for staff, minimise the
risk of adverse events, e.g. accidental extubation. Muscle relaxants/deep sedation be used while ventilating in prone position to avoid accidental displacement or extubation. First session of Prone ventilation is to at least 12 hours and further sessions much longer. Usefulness of prone position ventilation is shown by P/F >150, PEEP decreasing to <10, FiO2 decreasing to <0.6 after turning patient supine and that too lasting >4 Hrs
-
h. Fluid Management: Conservative fluid strategy to reduce extra vascular lung water.
-
i. Tracheostomy: Is an aerosolization procedure and so decide on clinical basis
-
j. Nebulisers: MDI preferred over nebulisers
-
k. Bronchoscopy: Diagnostic bronchoscopy is not recommended. ETA aspirates are adequate for RT PCR diagnosis of COVID
-
l. Liberation from MV: Standard weaning protocols with bridging to NIV with well fitted mask and dual limb circuits and strict airborne precau
SpO2<90% WITH OXYGEN>40% RESPIRATORY RATE>30 HEMODYNAMIC INSTABILITY
-
2. ECMO: Early evaluation & implementation.
-
i. ECMO Indications: Under optimal conditions
(FiO2>0.8, Tidal volume 6ml/kg PBW, PEEP>10 and no contraindication for prone ventilation) and prone ventilation has been implemented and meet one of the following
-
a. PaO2/FiO2<50 for more than 3 hours
-
b. PaO2/FiO2<80 for more than 6 hours
-
c. PaO2/FiO2<100 with FiO2=1
-
d. Arterial pH<7.25 and PaCO2>60 mmHg for>6hours
-
e. Arterial pH<7.20 and Plateau pressure >30cms H2O when Respiratory rate >35/minute
-
f. Concomitant cardiogenic shock or cardiac arrest
-
ii. ECMO contraindication:
-
a. Unrecoverable primary disease
-
b. Anticoagulation contraindicated
-
c. Mechanical ventilation > 7 days with higher settings (FiO2>0.9, Plateau pressure>30cm H2O), age older than 70 years, immunosuppression, presence of large peripheral vascular anatomy or disease
-
3. Choice of ECMO treatment: VV-ECMO. When circulatory failure of cardiac aetiology VA ECMO to be considered.
-
d. Drainage of airway secretions: Humidification with Heated humidifier /HME.CLOSED SUCTION device for Endotracheal suctioning.
-
e. Hemodynamic & Volume status: CONSERVATIVE FLUID STATERGY
-
i. Close monitoring of cardiac function: Echocardiography, Troponin T/I, NT BNP, Right heart function with ECHO.
-
ii. Tissue perfusion: monitoring and maintenance.
-
iii. Causes of shock in COVID sepsis include:
o Hypovolemia: Dehydration, Sepsis, Cytokine storm
o Vasoplegia
o RV failure: Due to ARDS, High ventilating pressure, Massive Pulmonary embolism (Assess by high CVP and with ECHO)
o COVID myocarditis: Assess with ECG, biomarkers,
Echocardiography
Find cause of hemodynamic instability (Systolic BP<90mmHg or 40 mmHg less than baseline, MAP<65mmHg, need for vasoactive drugs, severe arrhythmias) and treat the cause.
Arrhythmias should be actively managed.
Monitor clinically (Mental status, Urine output, capillary refill time, blood pressure, heart rate etc),functional hemodynamic monitoring like Passive leg raising test, Pulse pressure
variation, End expiratory occlusion, mini fluid challenge, Tidal volume challenge, IVC distensibility index, Echocardiography.
-
iv. Volume status: Do not overload the patient with adequate tissue perfusion. Small boluses of fluid are given and close monitoring of response noted. If signs of overload further fluids to be restricted. Over load can be assesses by worsening PaO2/FiO2, Extra vascular lung water if resources permit.
-
f. Nutrition: Early enteral feeds preferred (if no contraindications like dysfunctional gut, severe hemodynamic instability) with high calorie and proteins. 25-30 Kcal/kg/day. Protein: 1.5-2 gm/kg. Supplemental/Total parenteral if not tolerating/ dysfunctional gut.
-
g. STEROIDS: As mentioned in medical management of COIVD patient.
-
h. Antimicrobial: Routine use of antimicrobial is not recommended without clear evidence of bacterial infection.
-
i. Anticoagulant therapy: Mentioned in Medical management. UFH if abnormal RFT. Monitor coagulation profile &RFT
-
j. Sedation, Analgesia: Patient on Mechanical ventilation should be given appropriate sedation and analgesics
-
k. MUSCLE RELAXANTS: Routine use not recommended. If dyssynchrony can use Cis atracurium or atracurium.
-
l. Acute Kidney Injury & Renal replacement therapy: Second stage KDIGO criteria (Creatinine=2-2.9 times baseline value, urine output<0.5 ml/kg/hr for 12 hours) and other evidence for the need of renal replacement therapy-RRT
-
m. Infection transmission prevention:
-
i. Use of PPE as needed.
-
ii. HEPA BACTERIAL VIRAL filter placed at expiratory limb of ventilator tubing
-
iii. N95 mask / 3 ply mask for patient whenon Nasal Cannula for Oxygen supplementation.
-
iv. All intensive care personnel (medical, nursing, allied health, cleaning and ward assistants) receive training in infection control and personal protection equipment.
-
v. Recommend minimising aerosol generating procedures. If they must be performed, then they should be completed in a negative pressure room. If this is not available, then a single room should be used.
-
vi. When a unit is caring for a confirmed or suspected COVID-19 patient, ensure that all donning and doffing are supervised by an additional appropriately trained staff member.
-
vii. Recommend against the use of nebulised agents (e.g. salbutamol, saline) for the treatment of non-intubated COVID-19 patients due to the risk of aerosolization and transmission of infection to health care workers in the immediate vicinity.
-
viii. Clamp the ETT while intubating and when disconnection is required like during changing to transport ventilator.
-
ix. Allow time for complete muscle paralysis prior to intubation to avoid spontaneous exhalation by patient.
Aerosol generating procedures include:
o Intubation
o Extubation
o Bronchoscopy
o High flow nasal oxygen use
o Non-invasive ventilation (particularly with a poorly fitting mask)
o Procedures on screaming children
o Tracheostomy
o CPR prior to intubation
5. Transfer out of ICU:
Stable vitals, Oxygenation has improved (needs only Room air or low flow oxygen), weaned off from ventilator, Conscious, Respiratory rate<30/min, SpO2>93%, Stable hemodynamic, Not on support, No acute organ dysfunction.
-
IV. INTUBATION: Process & Precautions:
-
1. Ideally done in Negative Pressure room. If facility is not available intubate in single roomTreatment algorithms and cognitive aids needed should be displayed in the room.
-
2. Airborne precautions: For all staff in attendance: Fit check N95 mask, Goggles or face shield, Impervious gown, Gloves
-
3. Limit number of persons present at intubation site to 3, intubator, assistant and nurse.
-
4. Plans for difficult airway discussed beforehand.
-
5. Procedure to be done by the most qualified staff with the minimum number of health care personnel present as are required to undertake a safe intubation.
-
6. Video laryngoscopes should be used preferentially
-
7. Mac Coy Blades / intubating stylets use to minimise duration and easiness of intubation.
-
8. Oral Intubation preferred.
-
9. Preoxygenate-Spontaneous breathing with high FiO2 to minimise Bag mask ventilation
-
10. Use of viral filter on bag mask Circuit
-
11. Clamp endotracheal tube while intubating.
-
12. Post intubation positive pressure ventilation started only after inflating the ETT cuff and confirming position of ETT (ideally with EtCO2)
-
13. Endotracheal tubes with sub glottic suction aid preferred
-
14. Closed suction device to be used to prevent disconnections of circuit for removal of secretions
-
15. All intubation equipment including those for Difficult airway should be near the patient to prevent multiple exits& entry of health care personal.
-
V. TRANSPORT OF PATIENT:
-
1. Movement of patients with COVID-19 should be limited with all efforts made to ensure the patient is initially admitted to the appropriate location.
-
2. Non-intubated patients should be transferred wearing a surgical mask over their oxygen delivery device.
-
3. All staff must wear airborne PPE.
-
4. Once a patient is admitted to the ICU, transport outside of the ICU should be limited. If transport is required, then coordination at a senior level is mandatory to ensure safety standards are maintained
-
5. Hallways must be cleared where possible and only essential staff should accompany the patient. Staff not involved in the transfer should not come within 2 metres of the patient.
-
6. Intubated patients should have closed circuits with a viral filter in situ.
-
VI. PEEP: Optimal PEEP -one that has adequate oxygenation without affecting oxygen delivery to
tissues. (Does not affect hemodynamics)
-
• Step wise increment of PEEP at 2 increments monitoring hemodynamics.
-
• Static compliance method
-
1. Volume controlled Ventilation
-
2. Set pressure limit 10-15 cm H2O above ventilating pressure (or per institutional policy).
-
3. Turn ventilator sighs off for the procedure if being used.
-
4. Explain the procedure to the patient and to be as relaxed as possible.
-
5. Sedation sos
-
6. All measurements be obtained under the same conditions
-
7. Upright as possible.
-
8. Determine the static compliance at 0 cm PEEP.
-
9. The tidal volume, peak and plateau pressures should be noted.
-
10. Vt/Pplat-PEEP= Static compliance
-
11. Determine Static compliance at 3,6,9,12,15 cms H2O
-
12. The patient should be placed on the lowest PEEP level providing the greatest static compliance
-
VII. Recruitment: On a case to case basis. Methods:
-
1. 40cmH20 for 40-60 seconds
-
2. 3 consecutive sighs/min with a plateau pressure of 45cmH2O
-
3. 2 minutes of peak pressure of 50cmH2O and PEEP above upper inflection point (obese/trauma patients may require >60-70cmH2O)
-
4. long slow increase in inspiratory pressure up to 40 cmH2O (RAMP)
-
5. stepped increase in pressure (Staircase Recruitment Maneuver)
-
VIII. General care of Critically ill patients in ICU:
-
1. VAP prevention
-
2. Spontaneous Awakening & breathing Trials
-
3. Change in position 2Hourly
-
4. DVT Prophylaxis
-
5. Stress related mucosal disease prophylaxis
-
6. Nutrition
-
7. Psychological support
-
8. Debriefing
-
IX. Cardiac Arrest:AHA 2015 with modification to limit transmission
Recognise cardiac arrest. Look for absence of signs of life and normal breathing and feel for carotids. Do not listen or feel for breathing by putting your ear or cheek close to patient's mouth.
-
❖ Avoid mouth to mouth or pocket mask ventilation
-
❖ The staff should have gown, gloves, eyeshield or goggles before starting CPR (complete aerosol generating procedure PPE).
-
❖ Start CPR with chest compression.
-
❖ If patient is having oxygen mask before start of CPR leave it in situ to limit spread of aerosol. Otherwise if readily available put a mask and start CPR. Limit entry of people into the room during CPR.
-
❖ For bag and mask ventilation, connect HME or bacterial filter to it to limit aerosol generation. Use 2-person technique for bagging, one person to hold the face mask tight with E-V technique while the other ventilates to minimise aerosol generation.
-
❖ Identify and treat any reversible causes.
-
❖ Defibrillate shockable rhythms rapidly
Reference
-
1. WHO:Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected Interim guidance 13 March 2020
-
2. The Australian and New Zealand Intensive Care Society (ANZICS) COVID-19 Guidelines Version 1
-
3. Government of India Ministry of Health & Family Welfare Directorate General of Health Services (EMR Division) Guidelines on Clinical Management of COVID - 19
-
4. WHO:Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected Interim guidance 25 January 2020
-
5. Resuscitation council UK. Resuscitation of COVID - 19 patients in hospital. March 2020. (reference for cardiac arrest recommendations)
-
6. COVID-19 pneumonia: different respiratory treatment for different phenotypes? L. Gattinoni , D. Chiumello , P. Caironi , M. Busana , F. Romitti1 , L. Brazzi4 , L. Camporota
-
7. Gattinoni et al, 2020 - Covid-19 Does Not Lead to a “Typical” ARDS -INTENSIVE Review Aidan Burrell
FiO2-PEEP TABLE
Lower PEEP/hiqher FiO2
|
FiCh |
0 3 |
0.4 |
0.4 |
0.5 |
0.5 |
0.6 |
0.7 |
0.7 |
|
PEEP |
5 |
5 |
8 |
8 |
10 |
10 |
10 |
12 |
|
FiO2 |
0.7 |
0.8 |
0.9 |
0.9 |
• |
1.0 |
|
PEEP |
14 |
14 |
14 |
16 |
18 |
18-24 |
-
13. Interim Guidelines on Clinical Management of COVID 19 Infection In Children Coronavirus disease 2019 (COVD-19) caused by SARS COV 2 (severe acute respiratory syndrome coronavirus 2) is rarer in children compared to adults. Incidence of disease in children has been reported to be around 2% in most studies . Exact cause of lower incidence is not known. It may be due to lower susceptibility or higher incidence of asymptomatic disease in children. Nevertheless severe manifestations and deaths are being increasingly reported in children and they can act as an important source of infection for adults and health care workers as they cannot follow cough etiquettes as efficiently as adults.
COVID suspect
All symptomatic children (cough / sorethroat / URI / Diarrhea / shortness of breath with or without fever) who have
, a history of travel to or residence in a location reporting community transmission of COVID-19 disease during the 14 days prior to symptom onset.
, history of contact with suspected or confirmed case of Covid 19 in last 14 days prior to symptom onset.
, severe acute respiratory illness in the absence of an alternative diagnosis that fully explains the clinical presentation.
Testing
Indications for sending Nasopharyngeal swab for confirmation of Covid 19 Infection include
, Children suspected of having Covid infection, fulfilling the above criteria.
, Patients admitted with severe acute respiratory infection (SARI) irrespective of travel or contact history.
, Asymptomatic direct and high risk contacts of a confirmed case should be tested once between day 5 and 14 of coming in contact with the case (pooled RT - PCR).
, Children from containment zone with ILI.
, Children admitted in hospital with any other disease, who develop influenza like illness (ILI) in the hospital.
, Family clusters of ILI (2 or more cases)
, ILI in high risk population (children with comorbidities).
, Patients posted for elective surgeries (pooled RT - PCR) and emergency surgeries .
Covid 19 testing platforms
The preferred sample is nasopharyngeal. Nasal, throat swabs, ET aspirates and bronchoalveolar lavage may also be tested. The various methods of testing for Covid 19 include RT PCR: This is the gold standard for detecting Covid - 19 cases. It takes 4- 5 hours for running the test. Up to 96 samples are tested at time. Nasaopharyngeal swabs need to be transported in viral transport medium maintaining cold chain.
Pooled RT PCR: Pooled samples of 5 patients are tested by RTPCR. If positive then individual testing is done by RT PCR to identify and confirm disease in the affected. For this samples are collected in tubes containing 0.5ml of viral transport medium.
CBNAAT: It has a quick turnaround time of 30 - 60 minutes but only 1- 4 samples can be tested at a time. For CBNAAT, the samples may be sent in the standard viral transport medium. By far it is the costliest of the molecular tests for Covid.
TrueNat: It is an indigenously developed portable version of CB NAAT. It is the cheapest of the molecular based tests for covid - 19. Swab samples are collected in viral lysis buffer, hence biosafety and biosecurity requirements for use of TrueNat machine is minimal. It comprises of two steps (ICMR guidelines may 2020)
Step 1: screens for E gene. If negative it is considered true negative. If positive needs confirmation by step 2 assay.
Step 2: confirmatory assay for RdRp gene . All positives are taken as true positive and further confirmation by RT PCR not required.
Rapid antigen test: It is a point of care test which yields result in15 - 30 minutes. It sensitivity ranges from 50- 84% and specificity 99-100%. So those positive do not need a retesting, but those negative may not be real true negatives. Hence if suspicion is high, needs retesting with RT PCR.
IgG antibody test: It is only for surveillance and not for diagnosis. It helps to detect seroprevalance to plan public health interventions. May be done in high risk or vulnerable population to know who has been infected in the past.
Clinical Features
Incubation period ranges from 2 - 14 days, with a median time of 4-5 days. Asymptomatic and presymptomatic infection has also been reported. Clinical syndromes associated with COVID infection include mild uncomplicated illness with fever, sore throat, malaise, cough, diarrhoea or vomiting, mild pneumonia, severe pneumonia, ARDS, sepsis and septic shock with multi organ involvement. There is limited timeline data for infections in children. Adult literature suggests admission to hospital occurs approximately 7 days following symptom onset with onset of severe respiratory distress symptoms approximately 9 to 12 days after symptom onset. Covid illness most often starts with mild symptoms like dry cough and sore throat. 10% of patients may present with GI symptoms like diarrhoea and vomiting, while rhinorrhea is relatively rare (7.5%). Anosmia, ageusia and GI symptoms may precede development of respiratory symptoms. Patients may also complain of myalgia, headache, and fatigue. Fever and cough are seen less frequently in children than adults. Elderly and immunocompromised may present with atypical symptoms like fatigue, reduced alertness, reduced mobility, diarrhoea, loss of appetite, delirium, and absence of fever. Leucopenia is uncommon in children compared to adults. At admission 30% of symptomatic children may have leucopenia and 10 - 20% may have elevated CRP. Leucopenia and CRP > 10mg/dl has been found to be associated with pneumonia (12).
Clinical progression and complications
Clinical course may be hyper acute with rapid onset of fever and breathlessness or moderate with slower progression of symptoms and later recovery or biphasic with late progressive worsening and multi organ involvement. Illness severity can range from mild to critical. In study from china of > 44000 persons with Covid, 81% of infections were mild ( mild symptoms upto mild pneumonia), while 14% had severe symptoms like hypoxia, dyspnea or > 50% lung involvement in chest xray, while 5% had critical illness (respiratory failure, shock or multiorgan failure). Studies in children have reported fewer severe (5%) and critical illness (0.6%) (3)
Multisystem inflammatory syndrome in children (MIS-C) may occur weeks after a patient is infected with Covid-19(4). MIS - C should be considered in any individual less than 21 years of age presenting with fever with high inflammatory markers ( high CRP, ESR, Ferritin, Fibrinogen, D Dimer, LDH, IL- 6, elevated Neutrophils, low lymphocytes, Low albumin etc) with multi system (>2) organ involvement causing severe disease requiring admission, with no plausible alternative diagnosis and evidence of recent or past Covid infection as evidenced by positive RT PCR, antibody or antigen study or exposure to a suspected or confirmed Covid 19 case within the 4 weeks prior to admission.
Risk factor for severe disease
Age is an important risk factor. Case fatality rate is more in elderly, infants less than 1 year and in those with comorbidities like chronic lung, liver, kidney, neurological disease and in those with congenital or acquired immunodeficiency. Case fatality rate in children is less than 1%. Lymphopenia, neutrophilia, elevated SGOT, SGPT, LDH, CRP, Ferritin and D dimer is associated with more severe illness.
Reinfection and persistent RT PCR positivity
There is no conclusive data on possibility of reinfection with SARS COV- 2. Viral RNA shedding decreases with resolution of symptoms but may continue for days to weeks. Median range of viral shedding in hospitalised patient is 12 - 20 days. Presence of RNA during convalescence does not necessarily indicate viable infectious virus. Detection of IgM and IgG antibody often correlates with clinical recovery and immunity.
Triage
Hospitals should preferably establish a 3 tier triage system. At the point of first contact which is often the out-patient counter or hospital entry in case of non emergency patients, history of international travel or travel to hotspot areas with community trans- mission in the last 14 days or contact with suspected or confirmed cases should be elicited and all those with positive history should be directed to the Covid isolation area. Patients with positive history and their care takers should be offered a triple layer surgical mask and directed to the designated Covid isolation areas. In the Covid isolation areas the patients should be triaged for severity of infection.
Patient placement :
-
• Patients may be admitted in single rooms or in designated Isolation wards.
-
• There should be a double door entry with changing room and nursing station.
-
• All healthcare workers should use PPE (N95 mask, eye protection, gloves and gown) when entering a patient room and remove PPE when leaving.
-
• If possible, use either disposable or dedicated equipment (e.g. stethoscopes, blood pressure cuffs and thermometers). Equipment which is reused should be disinfected appropriately. Place an appropriate container with a lid outside the door for equipment that re- quires disinfection or sterilization.
-
• Avoid patient movement and transport unless absolutely necessary.
-
• Aerosol-generating procedures (i.e. open suctioning of respiratory tract, intubation, bronchoscopy, cardiopulmonary resuscitation) whenever possible, should be done in adequately ventilated single rooms , preferably negative pressure rooms with minimum of 12 air changes per hour or at least 160 litres/second/patient in facilities with natural ventilation. These rooms may have standalone air- conditioning. These areas should not be a part of the central air-conditioning. If air-conditioning is not available negative pressure could also be created through putting up 3-4 exhaust fans driving air out of the room. These procedures should be done after donning complete PPE, including gloves, long-sleeved gowns, eye protection, and fit tested particulate N 95 masks.
-
• Used PPEs should be disposed off as per the BMWM guidelines. Ensure these bins (dirty) are inside the isolation areas.
Clinical Management
All children with suspected COVID infection should be categorised into 3.
|
Category |
Clinical features |
Clinical severity |
Treatment |
|
Category A |
Mild sore throat, cough, rhinorrhea, diarrhoea, vomiting |
Mild |
Symptomatic treatment. Avoid NSAIDS other than paracetamol. Use oral bronchodilators or MDI for those with wheeze. Maintain adequate hydration. ORS and zinc for those with diarrhoea and vomiting |
Category B
Fever, severe sore throat, increasing cough Category A symptoms in children with chronic heart, kidney, lung, neurological or liver disease and children on long term steroids, congenital or acquired immunosuppression.
Mild
Oseltamivir 3mg/kg/dose BD till nasopharyngeal swab results are available if criteria for treatment of ILI fullfilled. Hydroxychloroquine 6.5mg/kg/dose BD on day 1 f/b 3.25mg/kg/dose BD for 4 more days Azithromycin 10mg /kg OD on day 1 followed by 5mg/kg OD on days 2 to 5. ECG should be taken prior to starting treatment to look for QT prolongation. zinc 2mg/kg/day.
Categorisation based on disease severity
Patients may be further categorised also based on disease severity according to the national guideline June 2020.
Mild: uncomplicated upper respiratory infection with fever, sore throat, rhinorrhea etc without breathlessness or hypoxia.
Moderate: Children with features of pneumonia without danger signs (Respiratory rate : < 2 months: > 60/mt ; 2-11 months: > 50/mt ; 1-5 years: > 40/mt ), spo2 90- 94%
Severe: spo2 < 90%, presence of danger signs like inability to feed, grunting, lower chest in drawings, altered sensorium, seizures etc.
Mildly symptomatic patients (Category A):
Patient placement: These patients can be sent home or to CFLTC with supportive treatment. They can also be treated at community health centres, district and sub district hospitals. They should stay away from elderly people, pregnant ladies, other children and patients with comorbidities (5). Clean frequently touched surfaces with 1% Sodium hypochlorite solution and toilet seats with household bleach or phenolic disinfectants. Wash linen separately with detergent and dry. Patient should follow strict personal hygiene including frequent hand washing and use of masks..
Testing
All category A patients with history of contact with a confirmed case of COVID 19 or coming from areas with community transmission should be tested for COVID 19 with nasopharyngeal swab RT PCR.
Treatment
-
• Symptomatic treatment: Avoid giving NSAIDS other than paracetamol for fever.
-
• Provide oral bronchodilators or MDI with spacer and mask for children with wheeze. Use of nebulisers should be avoided due to the risk of aerosolisation. Even though it is unclear if visible aerosols come from patients airway during nebulisation.
-
• Ensure euvolemia. Advice adequate fluid and feed intake. Provide advice regarding use of ORS and other home available fluids in case of diarrhoea and vomiting
-
• Categorisation should be reassessed every 24 -48 hours.
Category B patients
(patients with fever/increasing cough/ category A symptoms in patients with comorbidities)
Patient placement
These patients need admission. All category B patients should be admitted in single rooms or designated Covid isolation areas.
Monitoring
Daily monitor vitals including spo2, work of breathing and temperature.
Testing
Nasopharyngeal swabs must be sent for confirmation of disease by RT PCR in viral transport medium maintaining cold chain.
Chemotherapeutics
Children with ILI who fulfill the criteria for treatment can be started on Oseltamivir 3mg/kg/dose BD till nasopharyngeal swab results are available.. Antibiotics may be started as per treating physicians discretion if deemed necessary to cover community acquired pneumonia including atypical pneumonia according to local antibiogram. Once swab report is available and diagnosis confirmed Oseltamivir may be stopped and patient started on Hydroxychloroquine 6.5mg/kg/dose BD on day 1 followed by 3.25mg/kg/dose BD for 4 more days along with zinc 2mg/kg/day. (role of these chemotherapeutic drugs are still not proven and future guidelines may have a change in recommendation). Azithromycin 10mg /kg Od on day 1 followed by 5mg/kg OD on days 2 to 5. ECG should be taken prior to starting treatment to look for QT prolongation.
Moderate symptoms ( Category C)
Admit these patients preferably in dedicated Covid care hospitals, District hospitals, Medical colleges or other tertiary care hospitals catering to Covid patients.
Send nasopharyngeal swab for confirmation of Covid 19 infection.
Respiratory support
Give supplemental oxygen therapy immediately to patients with SARI and respiratory distress or hypoxaemia (Spo2<94%) .
Target Spo2 92- 96% for patients on oxygen therapy (6)
Nasal prongs or cannula are preferred in children as it may be better tolerated.
Offer surgical mask or hood covered by surgical mask to decrease risk of aerosolization and droplet spread.
If on prongs and SpO2 less than 92% with minimal respiratory distress, options include
-
a. Face mask at flow>5LPM ( Fio2 40 - 60%)
-
b. Oxygen hood at flow > 5LPM (Fio2 30-90%)
-
c. Venturi mask (28 -60% Fio2)
-
d. Non rebreathing mask at flow 10 - 15LPM ( Fio2 80 - 90%)
о Awake proning has been advised as a rescue therapy in adults.Typical protocols include 30120 minutes in prone position, followed by 30-120 minutes in left lateral decubitus, right lateral decubitus, and upright sitting position. Doing proning in children may be more difficult and needs close supervision
Investigations
For patients with respiratory signs and symptoms especially those on oxygen therapy, take chest xray. HRCT may pick up lesions before X ray but may not be feasible to take in all settings. Daily: 12 lead ECG, CBC with absolute lymphocyte count (Lymphopenia is rare in children), RFT, LFT ( SGOT, SGPT, PT INR, S Bilirubin).
Once in 48 to 72 hours: ferritin, D Dimer, CRP.
Monitoring
Monitor for worsening of symptoms like increased work of breathing, increasing oxygen demand or shock.
Drug treatment
Hydroxychloroquine 6.5mg/kg/dose BD on day 1 followed by 3.25mg/kg/dose BD for 4 more days along with azithromycin 10mg /kg OD on day 1 followed by 5mg/kg OD on days 2 to 5. ECG should be taken prior to starting treatment to look for QT prolongation.
zinc 2mg/kg/day.
If patient on oxygen support -
-
• Remedesivir 5mg/kg IV (max. 200mg) loading dose over 30 - 120 minutes on day 1 followed by 2.5mg/kg (max.100mg) IV OD on days 2- 4.
-
• Start methyl prednisolone IV 1mg - 2 per kg per day.
• Prophylactic low molecular weight heparin may be started if no contraindication at a dose of 1mg/kg s/c OD. In case of unavailability of remedesivir , Lopinavir/ ritonavir combination may be given along with HCQ.
Lopinavir ritonavir combination has not shown much promise as an effective treatment for Covid 19 infection. Favipiravir used in adults, is not yet licensed by DGCI for use in children. It is a teratogenic drug hence contraindicated in pregnancy. Favipiravir may be given in a case to case basis if deemed necessary after state medical board concurrence .
Category C patients (severe and critical disease)
Patient Placement
According to the severity of disease these children may.require either HDU or ICU care. Depending on the infrastructure, patient load and staff competence in managing sick children patient placement may vary from institution to institution. All children with moderate to severe ARDS ( P/F ratio less than 200 / OI >8 / OSI > 7.5 while on CPAP of minimum 5 cm), shock, multi organ involvement and those with Spo2 < 94% with increased work of breathing ( > 2site retraction/ paradoxical breathing / see saw breathing / head bobbing etc.) should be preferably admitted in PICU
Treatment of severe and critical patients
Monitoring
Vital signs: including heart rate, RR and Spo2, Blood pressure
Work of breathing: Watch out for increased work of breathing like retractions especially more than 2 site retractions, grunting, head bobbing, air hunger, large tidal volume breaths etc as these may indicate need for escalation of respiratory support inspite of having acceptable oxygen saturation.
Oxygen requirement: Monitor Oxygen requirement and provide appropriate oxygen delivery device. Target spo2 is> 94% during resuscitation and 92 - 96% for those on oxygen therapy.
Laboratory investigations
Routine investigations: CBC with differential count and ESR, CRP. Unlike adult patients with COVID-19 there have been no consistent leukocyte abnormalities reported in paediatric patients Organ functions: RFT, LFT, Coagulation Profile, ECG daily. Chest X-ray may show patchy infiltrates consistent with viral pneumonia and chest CT scans may show nodular ground glass opacities and evidence of peripheral consolidation which which may later progress to involve the whole lung fields.
Risk markers : CRP, D Dimer, Ferritin, Troponin I. Send these in all patients with severe or critical disease admitted in HDU or PICU every 48 hrs and in those with worsening respiratory status. D Dimer may be sent in in all those with hypoxia to look for evidence of microthrombi formation especially in pulmonary vasculature con- tributing to hypoxia. Elevated D Dimer is an independent risk factor for mortality in adults.
Hyperinflammatory syndromes
Some patients with Covid 19 infection may progress to multi organ failure due to hyperinflammatory syndromes like multi system inflammatory syndrome similar to Kawasaki disease, cytokine release syndrome or infection associated HLH often leading to multi-organ failure. Pointers towards hyper inflammatory syndromes include - Persistent high fever or reappearance of fever., rising CRP especially more than 100- 200 mg/L, doubling of ferritin in 24hours or very high ferritin levels (> 2000 - 10,000mcg/L), falling counts, rising or falling ESR, increasing CPK, LDH and new onset shock especially with elevated Top i/ TropT.
Tocilizumab:
Cytokine storm is an important cause of worsening of respiratory status with multi organ failure. Criteria for patients at high-risk for developing cytokine storm include Ferritin >300 ug/L with doubling within 24 hours , Ferritin >600 ug/L at presentation, CRP > 100 mg/L, LDH >250 and Elevated D-dimer (>1 mg/L) . Consider cytokine storm in patients with rapidly worsening respiratory gas exchange, radiographic infiltrates by imaging (chest x-ray, CT scan, etc.) and SpO2 <93% on room air or on greater than 6 L/min O2. Tocilizumab may be considered in patients with cytokine release syndrome grade 3 or 4.
Pediatric Dosing (<18yrs): < 30kg - 12mg/kg IV in 50 -100ml normal saline over 60 minutes > 30kg - 8mg/kg IV over 60minutes ( max. 800mg per infusion). Adult dose : 400mg IV over 60 minutes
Consider giving additional 2 dose 8-12 hours later if continued clinical decompensation Contraindications: Avoid in pregnancy and newborns. Mothers should stop breast feeding, if receiving tocilizumab Monitor liver enzymes in patients receiving tocilizumab. Serious adverse events: Gastrointestinal perforation, Anemia, Hepatitis, Infusion reaction
Steroids
Steroids may be considered in patients requiring oxygen support Dose: Methyl Prednisolone 1-2mg/kg/day for 5- 7 days. It may also be given to patients with grade 3 or 4 CRS with worsening respiratory status who cannot afford Tocilizumab. It may also be considered in patients with catecholamine refractory shock
Mulitisystem inflammatory syndrome in children: MIS-C has many similarities with KD but the median age of presentation is higher for MIS-C (10 years). GI symptoms like vomiting, diarrhoea and abdominal pain are often the initial presenting symptoms with high inflammatory markers. Some patients may present with features of acute abdomen. Many patients subsequently progress to develop cardiogenic shock in the next 5 -6 days. Global left ventricular systolic dysfunction is common but diastolic dysfunction, regional wall hypokinesis and right ventricular dysfunction has also been reported. Only around 17% of patients show coronary dilatation. Troponin I elevations are only mild to moderate in most patients. Skin rashes, neurological, renal and haematological manifestations can also occur.
Echocardiography, ECG, trop T/I, BNP or NT Pro BNP should be done in all suspected cases of MIS-C. History of immunodeficiency is risk factor for abnormal inflammation / HLH. Testing
Send nasopharyngeal swab for Covid 19 RT PCR. Serum sample should be taken for covid antibody test before IVIG administration and needs to be checked if facility available and RT PCR negative
|
^Multisys |
tem inflammatory syndrome in |
childrenl |
|
Clinical features |
Laboratory inflammatory markers |
Imaging |
|
Persistent fever Mucositis at any site Cervical lymphadenopathy Extremity changes Polymorphous rash Hepato-splenomegally Abdominal pain +/- diarrhoea ,vomiting |
high CRP (>100mg/dl), ESR, Ferritin, Fibrinogen, D Dimer, LDH, IL- 6, elevated Neutrophils, low lymphocytes, Low albumin , raised CPK, BNP/ NT pro BNP |
Echo: LV / RV dysfunction /regional wall hypokinesis/ pericardial effusion, valvulitis, coronary artery dilatation USG: colitis, ileitis, lymphadenopathy, ascites, hepatosplenomegaly CXR -patchy symmetrical infiltrates, pleural effusion. |
Treatment
Fluid resuscitation: resuscitate judiciously with normal saline 5 - 10ml/kg over 20mts if patient has features of shock, looking for features of fluid overload like hepatomegaly, respiratory distress, basal crepitations These children usually do not tolerate large volume fluids in excess of 20ml/kg.
After taking sample for blood culture start antibiotic to cover sepsis or TSS ( ceftriaxone + clindamycin)
Inotropic support as per physiological status. If BP maintained above 5th centile with poor perfusion Dobutamine may be started at 8 -10mcg/kg/minute and titrated.If patient hypotensive after fluid resuscitation titrate adrenaline 0.05- 0.3mcg/kg/mt. Respiratory support: Appropriate respiratory support may be provided as per childs respiratory and cardiovascular status.
IVIG 2gm/kg slow IV infusion may be started if MISC is diagnosed by clinical and laboratory markers. Sample for serology should be taken before starting IVIG. Methyl prednisolone may be indicated in refractory cases.Covid positivity is not a contraindication for use of steroids.
Respiratory support in severe cases
If a saturation of > 95% is achieved by flow of 15LPM oxygen, it indicates the shunt fraction is mild. Failure to achieve this indicates a moderate-severe shunt fraction. These children should be closely monitored for deterioration and respiratory support to be escalated as per need.
Heated humidified high flow nasal cannula (HFNC) may be used preferably over NIV if the target saturation is not achieved with routine 1st line oxygen delivery de- vices ( nasal prongs, NRM, nasal mask, Venturi , oxygen hood). It should be used only in patients with hypoxemic respiratory failure. It increases the risk of aerosolization but the risk is less than that for NIV.
-
• Switch on the machine only after fixing the nasal cannula.
-
• Start at 0.5 - 1litre per kg per minute and increase upto 2litre /kg/mt if needed.
-
• Use minimal flow that makes the baby comfortable
-
• Target spo2 92 - 96%
-
• Monitor HR and RR. Monitor closely. If no response in 1-2 hours will need escalation of support.
Jackson Rees with cushioned mask: It can provide oxygen at high fio2 and requires lesser oxygen flow than Baines circuit as dead space less. It also provides peep with minimal aerosolisation. It can be fixed using harness.
NIV CPAP: It may be offered only in selected patients with hypoxemic respiratory failure. Failure rate with NIV is very high especially in de novo respiratory failure so these patients need close monitoring.
-
• Use of conventional ventilators for NIV with non vented oro nasal masks / helmets preferable .
-
• Avoid using dedicated NIV with single limb and vented masks as the risk of aerosolization is very high.
-
• Connect a bacterial/ viral filter at exhalation port
-
• Use lowest possible PEEP to achieve targets
-
• Monitor closely for deterioration and intubate if patient deteriorates or there is no improvement in 1 hour or delivered tidal volume is more than 9.5ml/kg with increased work of breathing as P- SILI may damage the lung further
-
• Placing of aerosol box with ports covered by surgical mask may decrease risk of aerosolization.
Mechanical ventilation: it may be offered to children who do not respond to above respiratory support interventions. Indications include
, Spo2 less than 90%/ P/F ratio less than 150 not responding to above measures
, Severe respiratory distress including high tidal volumes of more than 9.5ml/kg in NIV
, Refractory shock
, Altered mental status with GCS less than 8
Airway management
Airway management should be SAS (safe, accurate, swift). Safe for patient and staff, Accurate, avoiding unfamiliar, unreliable and repeated techniques and Swift i.e timely without rush or delay.
-
• There is no emergency intubation in pandemic
-
• Unplanned intubation will harm both pa-ent and health care worker increasing risk of spread of infection and worse outcome.
-
• Perform intubation only after donning complete PPE
-
• Limit number of persons present at intubation site to 3 . Intubator, assistant and Nurse.
-
• If facility available intubate in a negative pressure room with > 12 air changes per hour or 160 litres /second / pa-ent in areas with natural ventilation and then shift to main ICU preferably with the same facility to minimise aerosol genera-on and expo- sure to others.
-
• Treatment algorithm and cognitive aids needed like ET tube size, fixing length etc should be displayed in the room.
-
• All drugs needed should be preloaded preferably outside the room. Adrenaline 0.1ml per kg 1 in 10,000 solution
Atropine 0.02 mg/kg (not needed as routine, use in case of bradycardia or as antisialogogue before ketamine or if using succinylcholine especially repeat dose) Ketamine 1- 2mg/kg
Rocuronium 1. 2mg/kg or succinylcholine 1mg/kg
Midazolam 0.2mg/kg
Fentanyl 2mcg/kg or Morphine 0.1mg/kg
-
• Ensure full neuromuscular blockade before attempting intubation to limit aerosol generation and clamp ET tube before intubation.
-
• Use aerosol box or intubate under transparent sheet to minimise aerosol generation.
-
• Fluids and inotropes may be started if hemodynamically unstable before intubation.
-
• Intubation is preferably performed by the most experienced person to minimise exposure and attempts.
-
• Preoxygenate preferably allowing spontaneous breathing with NRM , anaesthetic bag or Jackson Rees with face mask for 3 minutes. . Manually bag only if respiratory efforts poor or oxygenation not maintained.
-
• If bag and mask ventilation needed. Connect HME or bacterial filter to it to limit aerosol generation. Use 2 person 2 handed technique for bagging, one person to hold the face mask tight while the other ventilates to minimise aerosol generation by decreasing leak.
-
• Video laryngoscope is preferred if available over direct laryngoscope for intubating children with suspected COVID infection.
-
• Connect ventilator tubings to the ventilator with bacterial / viral filter at exhalation port and set the initial settings beforehand. Inline closed suction and HME filter if being used should also be connected to the tubings beforehand. Post intubation inflate cuff and baby may be directly connected to the ventilator tubings without bagging if possible.
-
• Check position of ET tube by clinically looking for chest rise , ET co2 if available and check Xray.
-
• Clean room 20 minutes after aerosol generating procedure or intubation done.
-
• If intubation failed after 3 attempts 2nd generation supra glottic airways like proseal LMA may be used if available.
-
• Place NG tube after intubation and ventilation established safely.
-
• Closed suction preferred over open suction in ventilated pa-ents. Use in-line catheters for airway suctioning and clamp endotracheal tube when disconnection is required (eg.transfer to a transport ventilator).
-
• Post intubation endotracheal sample should be taken for testing if needed (preferred over nasopharyngeal sample).
Supportive care
Keep patient in semi recumbent position and change position every 2 hours. Stress ulcer prophylaxis should be offered with sucralfate or PPI in patients with risk of bleeding. Change heat and moisture exchanger every 5 - 7 days, when it malfunctions or is soiled. Adult HME filters should not be used for small babies as it will increase dead space
Initial Ventilator setting
PCV mode is preferred in children. Titrate Fio2 to target spo2 of 90 - 94% or PaO2 60 - 80 mm of Hg. Pressure support over PEEP or PIP should be kept at a level that delivers tidal volume of 8 ml/kg predicted body weight for height to start with then decrease to achieve 6ml/kg TV if compliance poor. Keep initial PEEP at 6cm later can be titrated.
Ventilation goals
SpO2 90 - 94%, pH > 7.3 , P plateau < 28 (<31 if chest wall oedema plus) and driving pressure less than 15 cm H2O. If P plateau more than 28, decrease TV to 4-6ml/ kg predicted body weight. TV of 8ml/kg may be acceptable in L type phenotype with normal compliance especially if there is asynchrony or pH less than 7.15.
Covid 19 pneumonitis
The etiology of Hypoxia in Covid infection is multifactorial. It may be due to ventilation perfusion mismatch due to hyperperfusion of lungs initially followed by pulmonary thrombophlebitis causing pulmonary thrombosis in later stages of disease. Classical ARDS type of lung pathology due to damage to basement membrane and secondary surfactant deficiency is also a cause of hypoxia in certain patients.
Identification of type of lung.
The two types of lung are not mutually exclusive. They often indicate 2 ends of the same spectrum. The lung which initially starts as L type often progresses to H type as the disease progresses. Ventilation strategies differ according to the type of lung. These 2 phenotypes are not mutually exclusive, they may indicate lung in different stages of evolution of disease. Increased work of breathing contributes to lung damage by increasing patient self inflicted lung injury (P- SILI) and is responsible for transition from L Type to H Type (8).
L type lung
This lung type with good compliance and hypoxia due to ventilation perfusion mismatch is characterized by upright pressure volume loops, attainment of good tidal volumes by low PIP, well aerated lung in USG with A lines.
These patients can be ventilated at 6 - 8ml/kg TV and PEEP 6 - 8cm of H2o. If hypoxia persists prone ventilation may be considered. Higher titration of PEEP is not beneficial as amount of recruitable lung is low. Low molecular weight heparin 1mg/kg Sc OD may be started if there is no contraindication and after assessing risk of bleeding.
H type lung
This is the classical ARDS lung with low compliance characterized by low lying Pressure volume loops, closed flow scalars, need of high pressures to attain 6ml/kg TV. In these patients PEEP may be titrated looking at compliance and hemodynamic status. Refractory hypoxia should be managed with titration of PEEP and prone ventilation of 12 - 16 hrs /day. Recruitment manoeuvres though not routinely recommended may be tried in refractory cases at 30cm of H2O for 15 seconds after ensuring absence of air leak and patient should be closely monitored during procedure for hemodynamic compromise.
Proning
Consider proning if P/F ratio <150 while being ventilated with FiO2 >0.6 and PEEP >5 cm H2O. Keep prone for 16 -18 hours if possible.
Shock
Any hypotension (systolic blood pressure [SBP] < 5th centile or <70 + age X2 for 1- 10 year old or > 2 SD below normal for age or cold extremities with capillary refill > 3 s and a weak and fast pulse.
• Give 10-20 mL/kg crystalloid (NS/RL/PL) as a bolus in the first 30-60 minutes and reassess for signs of fluid overload after each bolus [1]. If cardiogenic shock is suspected give careful fluid bolus of 5 -10ml/kg over 30 Mts looking for features of fluid overload like increase in liver size, basal crepitations or worsening respiratory distress.
-
• Look for evidence of cardiac injury in patients with shock with clinical examination, bedside echo and cardiac enzymes Trop T or trop I.
-
• Do not use synthetic fluids for resuscitation
-
• Do not use albumin as the initial fluid for resuscitation.
-
• Epinephrine or Norepinephrine may be used as the initial inotrope (in adults Norepinephrine preferred while in children adrenaline is the initial inotrope of choice) in fluid refractory shock. Further titration depends on hemodynamic parameters.
-
• Noradrenaline may be added to adrenaline if hypotension persists inspite of fluid and adrenaline.
-
• In patients with evidence of cardiac dysfunction and persistent hypoperfusion in spite of fluids and norepinephrine add dobutamine over increasing dose of nor- epinephrine.
-
• In case of catecholamine refractory shock low dose hydrocortisone 2- 4mg/kg/ day may be used as continuous infusion or intermittent dose.
-
• Specific therapy: No specific antiviral therapy is definitely proven to be effective as per current available literature. Drugs being used in clinical trial settings include
-
► Hydroxychloroquine / Chloroquine
-
► Remedesivir
-
► Nitazoxanide
-
► Ivermectin
-
► Favipiravir
Hydroxychloroquine : 6.5mg /kg /dose (Max 400mg) PO BD day 1 followed by 3.25mg per kg PO BD (max 200mg/dose) for 4 days. Treatment duration: 5 days. In select patients with extended ventilation or profound immunosuppression duration may be extended. Avoid taking hydroxychloroquine with antacids. Separate administration by at least 4 hours Adverse events: Retinopathy, rash, nausea, glucose fluctuations, and diarrhea. GI symptoms can be mitigated by taking hydroxychloroquine with food. Contraindications: QT prolongation > 500 msec, porphyria, myasthenia gravis, retinal pathology, epilepsy. If baseline QT prolongation is present take frequent ECG.
Chloroquine: Hydroxy chloroquine is preferred over chloroquine. 10 mg /kg chloroquine sulphate base stat followed by 5mg per kg 12 hours later and then 5 mg / kg/ dose BD for 4 more days. Adult Dose: Chloroquine sulphate base 600mg (10mg/kg) stat followed by 300 mg 12 hour later followed by 300mg BD for 4 days.
Lopinavir / Ritonavir: May be considered in case to case basis after written consent and medical board concurrence in category C cases when Remedesivir is not available. Presently in adults favipiravir preferred over lopinavir in moderate cases .
14 days to 6 months : 16mg/kg/dose PO BID ( based on lopinavir component)
< 15kg : 12 mg/kg/dose PO BID (based on lopinavir component)
15-25 kg: 200 mg/50 mg PO BID
26-35 kg: 300 mg/75 mg PO BID
>35 kg: 400 mg/100 mg PO BID
Adult dose : 400/100 PO BID
Duration of treatment: 14 days or 7 days after becoming asymptomatic.
Adverse events: Hepatotoxicity, pancreatitis, diabetes, QT prolongation, lipid elevations.
Remdesivir
EUA (emergency use authorisation) has been granted by FDA for use in children and adults with severe disease. It is yet to be approved by DGCI for use in children, hence should be administered only after written consent and medical board concurrence. Indicated in category C patients with moderate and severe illness on respiratory support.
Dose : 5mg/kg IV (max. 200mg) loading dose over 30 - 120 minutes on day 1 followed by 2.5mg/kg (max.100mg) IV OD on days 2- 4.
Duration of treatment: Usual duration 5 days. If no clinical improvement, duration may be extended to total 10 days.
Contraindications: AST/ALT > 5 times above upper limit of normal. Severe renal impairment, pregnancy and lactation.
Convalascent plasma
It may be considered as per state protocol for patients with moderate and severe disease. It is an off label use. It should be avoided in patients with IgA deficiency or immunoglobulin allergy. According to ICMR guidelines ABO compatible cross matched plasma with neutralising titre above the threshold level or plasma IgG titre against S - protein RBD above 1: 640 should be used.
Dose 10ml/kg.
Follow up sample and discharge
1st follow up sample is to be taken on the 10th day from the 1st day of positivity for asymptomatic and category A patients. For category B and C patients 1st follow up sample to be taken on the 14th day from the 1st day of positivity.
If test remains positive on day 14 repeat testing every alternate days till negative. The patient may be discharged if he/she has completed 14 days since onset of symptoms and no symptoms since last 3 days and test is negative.

Designed by Dr. Serin Kuriakose © All rights reserved 2020. Contact us at admin@afpikerala.in

 Government Orders
Government Orders