Coronavirus Disease 2019 - COVID 19
COVID-19: Rapid Testing
Protocol for using 'Rapid antibody test'in Hot area - epidemiological studies and surveillance
Secretary to the Government of India
Letter to Addi.Chief Secretaery/Secretary/Principal Secretary Health (All States)
Sub: Protocol for using 'Rapid antibody test' in Hot area - epidemiological studies and surveillance
Department of Health Research
Ministry of Health & Family Welfare &
Director-General, ICMR
Dated: 17th April 2020
I am writing to you with reference to the rapid antibody test kits for COVI D-19 testing. It is understood that many States intend to use these kits in affected areas.
2 The National Task Force at ICMR has carefully reviewed the data evolving from various countries on use of such kits. Based on available evidence, the testing strategy for COVID-19 has been revised further. The revised document is enclosed for your reference .
3. It is critical to understand the following key facts while using the rapid antibody tests:
- Gold standard frontline test for COVI D-19 diagnosis is real time PCR based molecular test, which is aimed at early virus detection .
- The rapid antibody test cannot replace the frontline test.
- The rapid Antibody test is a supplementary tool to assess the prevalence of the diseases within a specific area I perimeter.
- The rapid antibody test will only be of utility after a minimum of 7 days of onset of symptoms.
- Data about these rapid tests is emerging and understanding of their utility for diagnosis is still evolving.
- The rapid tests are useful for epidemiological studies and surveillance purposes.
- THE TEST HAS TO BE DONE UNDER STRICT MEDICAL SUPERVISION.
4. The enclosed ICMR advisory is for Hot spots. In case your state does not have a Hot spot, these tests may be used for:-
- Any hotspot which may emerge in future OR
- As a surveillance tool for epidemiological purposes in such areas where cases have not emerged so far.
5. Before starting the rapid test, it should be registered on covid19cc.nic.in/ICMR and data related to the test should be reported on the same.
A. COVID-19 Testing Strategy for India (Recommended for the entire country)
Real-Time PCR (RT-PCR) test and Point-of-Care molecular diagnostic assays are recommended for diagnosis of COVID-19 among individuals belonging to the following categories:
- All symptomatic individuals who have undertaken international travel in the last 14 days
- All symptomatic contacts of laboratory confirmed cases
- All symptomatic health care workers
- All patients with Severe Acute Respiratory Illness (fever AND cough and/or shortness of breath)
- Asymptomatic direct and high-risk contacts of a confirmed case should be tested once between day 5 and day 14 of coming in his/her contact
B. Additional (in addition to A) Testing recommended in hot spots

Recommendations dated :27th March 2020
Diagnostic testing for SARS-CoV-2 is extremely important for ifs control. It is those countries that have tested the most people that have been able to contain the spread and mortality. When considering which test to use, we have to take into account the accuracy, cost, infrastructure and human resource available. The following approach may be considered.
1. Use PCR as the primary diagnostic modality
2.- Set up testing facilities using Real Time PCR in as many centres as possible. The tests maybe done in clinical and research laboratories that routinely offer PCR based tests. Machines from universities etc may be borrowed on a temporary basis to boost throughput.
- Point of care cartridge PCR may be used in centres where it is available. This would be more useful in the peripheral centres like the TB control units
- Private laboratories may be asked to contribute to the effort by helping to test the pool of patients identified by the public health authorities. They should be supplied reagents free for this purpose.
- Free market testing may be discouraged as it will mainly be used by hypochondriacs with money to spare and waste resources.
Role of antibody based tests
Antibody tests need not be used as a primary diagnostic test.
- It can however be used sparingly as an adjunct in doubtful cases negative for PCR and fo fest contacts for epidemiological purposes as needed.
- The main use of the antibody test would be to study the incidence and prevalence of disease and local outbreaks by well-designed studies setting up surveillance centres.
- They can be used in a limited manner to screen new arrivals from within or outside the country and those who test positive may be quarantined. Efforts should be obtained to procure FDA/ICMR approved rapid test kits which will help in disease surveillance.
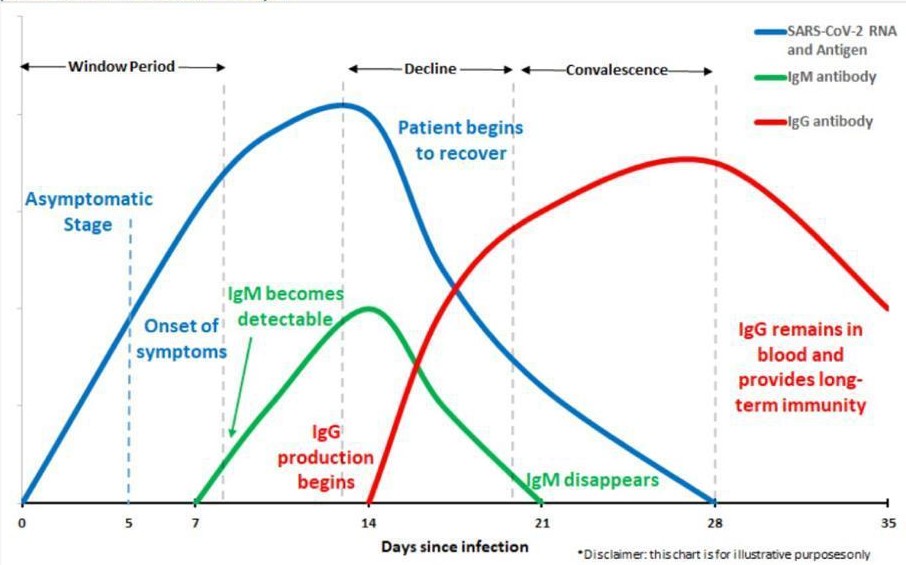
|
|||
|---|---|---|---|
| PCR | IgM | IgG | Clinical significane |
| + | - | - | Patient may be in the window period of infection |
| + | + | - | Patient may be in the early stage of infection |
| + | + | - | Patient is in the active phase of infection |
| + | - | + | Patient may be in the last / recurrent stage of infection |
| - | + | - | Patient may be in the early stage of infection. PCR may be false negative. |
| - | - | + | Patient may have had a past infection and has recovered. |
| - | + | + | patient may be in the recovery stage of infection, or the PCR result may be false negative. |
THE ELIGIBILITY OF PRIVATE and GOVERNMENT LABORATORIES
- NABL accredited laboratories
- Laboratories approved by ICMR to do COVID 19 testing.
- They will be given a dossier for online registrafion and online reporting of the test results. For any clarification and initial registration they may send the request on the following email address covidpsnodedme@gmail.com
- Use of Rapid Test Kits approved by FDA or ICMR only.
- Detailed guidelines for the Laboratories are issued below.
Criteria for selection of a patient /person for COVID-19 Rapid Antibody Testing
- The patient /person should have a prescription for COVID-19 testing issued by a registered medical practitioner.
- Tests can be performed on persons who have returned from foreign countries or the contact of persons returned from the foreign countries.
- Tests can be performed in COVID-19 suspects or symptomatic high risk contacts that were negative by RT-PCR.
- Tests can be performed in high risk individuals like healthcare workers who work in COVID-19 designated treating facilities who are involved in direct care of COVID positive patients.
- Tests can be done in a locality where a cluster of Severe Acute Respiratory Infection (SARI) cases without a diagnosis has been reported.
- Tests can be performed on individuals who have recovered from SARI without a diagnosis.
SOPs for Management & Reporting Rapid Antibody Test for COVID-19 by the Laboratories:
- The laboratories should have a valid NABL accreditation (Quality Council of India).
- A Medical officer from the Laboratory should be identified as a nodal person for communications related to Rapid Diagnostic Testing for COVID-19.
- The laboratory should register online for getting approval for initiating Rapid Diagnostic Test for COVID-19. The facilitation will be provided through the email ID covidpsnodedme@amail.com on request.
- Once they obtain registration from Govt, of Kerala they can start doing the Rapid Diagnostic Tests for COVID-19.
- ONLY United States Food and Drug Administration (US FDA) or Indian Council For Medical Research (ICMR) approved Rapid Antibody kits for COVID-19 should be used for testing.
- The testing should be done only with a prescription from a registered Medical Practitioner.
- The category of people on whom Rapid Antibody Test can be done is as mentioned above.
- All COVID-19 transmission based precautions should be taken into consideration while taking the samples, testing and while in contact with the patient. Full personal protective equipment should be worn before sampling, separate COVID testing corner without overcrowding should be ensured and mixing of suspected patients with other customers should be avoided. Good Laboratory Practices should be observed at all points.
- Training for all personnel involved in the process (from COVID reception area, sampling, testing including security personnel) should be trained in prevention of COVID-19.
- The test results should be reported online (real-time) in the format given in the annexure-1. The access to the online reporting portal shall be provided once the registration is complete.
- .The Nodal person in charge of COVID-19 rapid antibody testing should ensure confidentiality and privacy of reporting of results, safety of data, Good Laboratory Practices and the online facility provided for online reporting only.
- Guidance on Rapid antibody kits for COVID-19 and availability of antibody based rapid kits approved by CE -IVD and ICMR are available on the ICMR website released on 27th March 2020. https: //icmr.nic.in/sites/default/files/uoload documents /Guidance on RapidKits COVID19 27032020.pdf
COVID 19 (nCorona) Virus Outbreak Control and Prevention State Cell
Health & Family Welfare Department
Government of Kerala
COVID-19 - ADVISORY ON USING RAPID DIAGNOSTIC KITS FOR COVID-19
DIAGNOSIS AND SURVEILLANCE - Reg
No.31/F2/2020/Health — 27th March 2020

Designed by Dr. Serin Kuriakose © All rights reserved 2020. Contact us at admin@afpikerala.in

 PPE requirement calculator
PPE requirement calculator